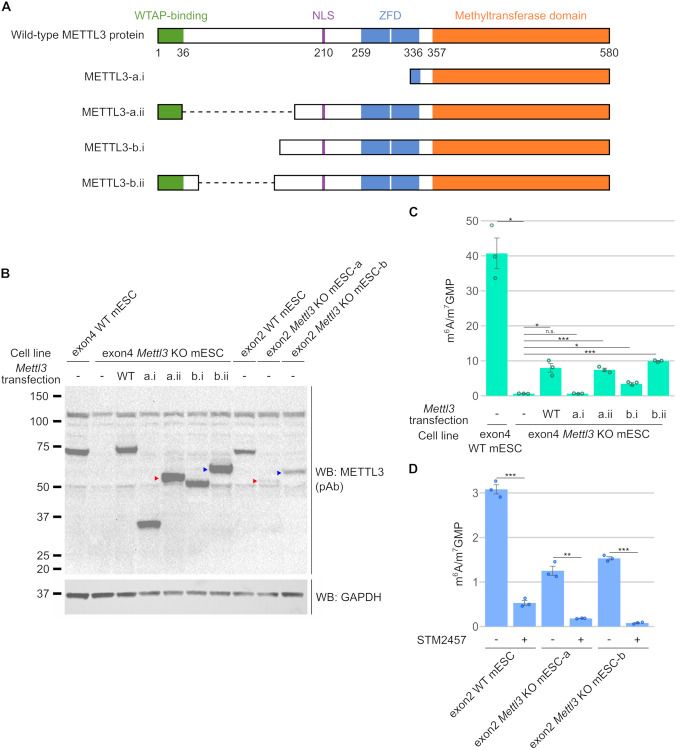
Fig 2. Shortened isoforms of METTL3 catalyze m6A formation in Mettl3 KO mESCs.
(A) The predicted domain structure of proteins encoded by the altered METTL3 ORFs expressed in exon2 Mettl3 KO mESCs suggests they may be functional. The domains that are known to be necessary for m6A formation by METTL3 include the WTAP-binding domain [39], ZFD [19,36], and methyltransferase domain [19,20]. To determine if the METTL3 ORFs from exon2 Mettl3 KO mESCs encode functional METTL3 proteins, we predicted the domain structure of the METTL3 protein isoforms from their ORFs. While all the predicted proteins have the methyltransferase domain, only METTL3-a.ii and METTL3-b.ii have all the known critical domains for m6A-writing. (B) WB of transfected FLAG-tagged METTL3 isoform ORFs. To determine if the METTL3 ORFs we found in the knockout cells can synthesize m6A, we expressed the METTL3 ORFs in exon4 Mettl3 KO mESCs that exhibit no METTL3 protein and essentially no baseline m6A signal. After 48 h, the alternatively spliced METTL3 proteins can be detected by immunoblotting with an anti-METTL3 antibody. FLAG-METTL3-a.ii (50 kDa, red arrowhead) and FLAG-METTL3-b.ii (55 kDa, blue arrowhead) have similar sizes to the anti-METTL3-antibody-reactive protein seen in exon2 Mettl3 KO mESC-a (red arrowhead) and exon2 Mettl3 KO mESC-b (blue arrowhead), respectively. 30 μg per lane. (C) Isoforms of METTL3 proteins can write m6A. After 48 h of transfection, RNA from each sample was processed, and m6A was measured using mass spectrometry. Expression of full-length WT METTL3 was able to rescue 19.7% of the m6A. METTL3-a.i was unable to rescue m6A, but METTL3-a.ii was able to rescue 18.3% of m6A. METTL3-b.i could only rescue 8.5% of m6A, whereas METTL3-b.ii rescued 24.4% of m6A, respectively. Thus, METTL3-a.ii and METTL3-b.ii proteins that are expressed in the exon2 Mettl3 KO mESCs are able to catalyze the formation of m6A. Error bars indicate standard error (n = 3). * = p-value < 0.5, ** = p-value < 0.01, *** = p-value < 0.005, n.s. = not significant. Underlying data can be found in S1 Data. (D) A METTL3-specific inhibitor leads to loss of m6A even in exon2 Mettl3 KO mESCs. Exon2 WT and Mettl3 KO mESCs were treated with 30 μM STM2457, a METTL3-specific inhibitor, and m6A levels were measured by mass spectrometry after 48 h. STM2457 treatment reduced m6A by 82.8% in the WT mESCs. In exon2 Mettl3 KO mESC, m6A was reduced by 85.4% in Mettl3 KO mESC-a after STM2457 treatment and by 94.8% in Mettl3 KO mESC-b. Thus, METTL3 is responsible for the remaining m6A in the exon2 Mettl3 KO mESCs. Residual m6A after STM2457 treatment may reflect incomplete inhibition of METTL3 at 30 μM. Error bars indicate standard error (n = 3). * = p-value < 0.5, ** = p-value < 0.01, *** = p-value < 0.005, n.s. = not significant. Underlying data can be found in S1 Data. m6A, N6-methyladenosine; mESC, mouse embryonic stem cell; NLS, nuclear localization signal; ORF, open reading frame; pAb, polyclonal antibody; WB, western blot; WT, wild-type; ZFD, zinc finger domain.