Table 2.
Different PARP Inhibitors Comparison and Summarized Clinical Benefits
| PARP Inhibitors | Olaparib | Rucaparib | Niraparib | Talazoparib | Veliparib | Pamiparib | Fluzoparib |
|---|---|---|---|---|---|---|---|
|

|
|
|
|
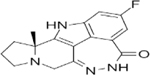
|

|
|
| IC50 (nM) | 1 | 1 | 4 | 0.6 | 2 | 0.83 | 1.5 |
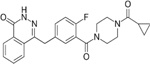
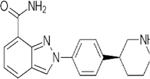
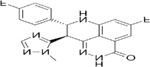
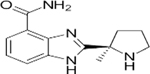
| Targets | PARP1/2/3/4 | PARP1/2/3/4, TNKS1 | PARP1/2 | PARP1/2 | PARP1/2/3 | PARP1/2 | PARP1/2 |
| Trapping capacity [1–5] | ++ | ++ | ++ | +++ | - | ++ | N.A. |
| Single-agent dose | 300 mg PO BID | 600 mg PO BID | 300 mg PO BID | 1 mg PO QD | 400 mg PO BID | 60 mg PO BID | N.A. |
| CNS penetration | + | + | ++ | - | + | ++ | N.A. |
| T1/2 (hours) | 11.9 | 25.9 | 48–51 | 90 ± 58 | 4.1–8.2 | N.A. | N.A. |
| P-glycoprotein substrate | Yes | Yes | Weak | Weak | No | No | N.A. |
| Off-target interactions | CYP3A4 | CYP enzymes | Dopamine, norepinephrine and serotonin transporters | None | N.A. | N.A. | N.A. |
| Toxicities (most frequent) | Nausea, fatigue, vomiting, diarrhea, dysgeusia, headache | Nausea, fatigue, vomiting, diarrhea, dysgeusia, LFT elevation | Nausea, fatigue, LFT elevation, vomiting, headache, insomnia, HTN | Nausea, fatigue, headache, vomiting, alopecia, diarrhea | Nausea, fatigue, lymphopenia | Nausea, fatigue | Limited early-phase trial data |
| Grade higher than 3 hematologic toxicities | Anemia, neutropenia | Anemia, neutropenia | Thrombocytopenia, anemia, neutropenia | Anemia, neutropenia, thrombocytopenia | NTD | Anemia, neutropenia | NTD |
| Clinical benefits | SOLO1 (ovarian maintenance), HR 0.30, PFS benefit; SOLO2 (relapsed ovarian maintenance), HR 0.30, PFS benefit; OympiAD (HER2 BC), HR 0.58, PFS benefit | ARIEL2 (relapsed ovarian), HR 0.27, PFS benefit; ARIEL3 (relapsed ovarian maintenance), HR 0.23, PFS benefit | NOVA (relapsed ovarian maintenance), HR 0.27, PFS benefit | EMBRACA (HER2 BC), HR 0.54, PFS benefit | BROCADE 3 (advanced BC), HR, 0.71, PFS benefit | Ongoing, data not mature (NCT03427814) | NTD |
| Approvals | Ovarian, Breast | Ovarian | Ovarian | Breast (FDA) | NTD | NTD | NTD |
CNS, central nervous system; CYP, cytochrome P450; IC50, half maximal inhibitory concentration; N.A., not available; PARP, poly(ADP-ribose) polymerase; TNKS1, tankyrase 1; -, poor; +, modest; ++, good; HR, hazard ratio; PFS, progression-free survival
