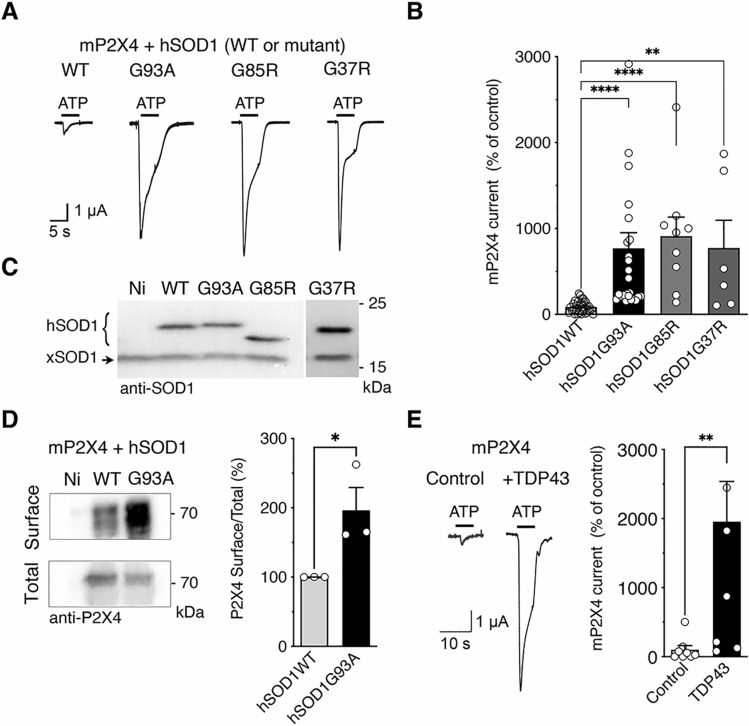
Fig. 1.
Mutant SOD1 proteins increase surface P2X4 number and function in vitro. A Representative currents evoked by 100 µM ATP in Xenopus oocytes co-injected with cDNAs encoding the murin (m)P2X4 and either the wild-type (WT) human SOD1 (hSOD1WT) or a mutant hSOD1 (hSOD1G93A, G85R or G37R). B Mean amplitudes of ATP induced-currents computed for all tested oocytes. ATP evoked P2X4 currents are strongly increased in cells expressing mutant hSOD1 (G93A, G85R and G37R) compared to those expressing hSOD1WT (** p < 0.01, ***p < 0.001, one-way ANOVA). C Western blot using anti-SOD1 antibodies confirmed the expression of the WT or mutated hSOD1 protein in addition to the endogenous Xenopus SOD1 (xSOD1) present also in non-injected oocytes (Ni). D Left, western blotting of surface and total proteins purified after protein surface biotinylation using anti-P2X4 antibodies from oocytes co-expressing mP2X4 and hSOD1WT or hSOD-G93A and non-injected oocytes (Ni). Right, normalized surface/total ratio shows that surface density of mP2X4 is increased in cells expressing SOD1G93A compared to those expressing hSOD1WT (*p < 0.05; unpaired t test). E Representative currents evoked by the application of 100 µM ATP in Xenopus oocytes co-expressing mP2X4 and human TDP43. Mean amplitudes of ATP induced currents computed for all tested oocytes (**p < 0.01; unpaired t test). Values of each cell or independent experiment are indicated on the graphs