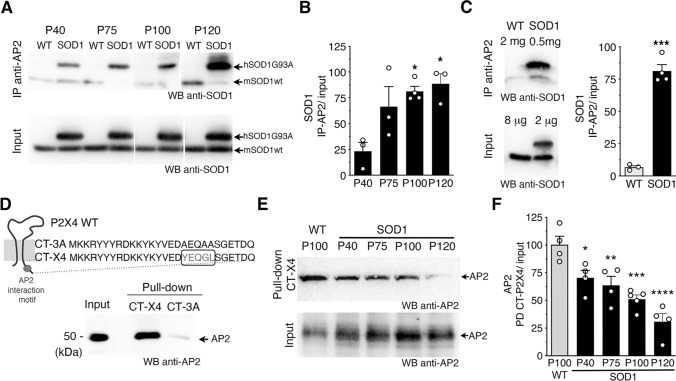
Fig. 2.
Mutant SOD1 proteins alter AP2 dependent endocytosis of P2X4 over ALS progression in the SOD1 mouse model. A Western blot analysis using anti-SOD1 antibodies after immunoprecipitation (IP) using anti-AP2 antibodies from spinal cord protein extracts of wild type (WT) and SOD1 mice at different stages (P40 to P120) revealed that SOD1-G93A co-immunoprecipitated with adaptor protein 2 (AP2) (see also panel E and Fig. S1B-C). Anti-SOD1 antibodies revealed in total proteins (input) one (mSOD1) or two bands (mSOD1 + hSOD1G93A) confirming the genotype of the mice tested. B The increase in SOD1 signals after IP over time suggests that the interaction between SOD1-G93A and AP2 increases during ALS pathogenesis (significantly different from P40, *p < 0.05, one-way ANOVA). C Co-IP control experiments performed with fourfold more proteins extracted from spinal cords of WT to reach similar amount of endogenous mSOD1WT and hSOD1G93A. SOD1 signals after IP with anti-AP2 show that solely SOD1G93A co-immunoprecipitated with AP2 (***p < 0.001, unpaired t test). D Pull-down assay using an immobilized peptide coding for the C-terminal domain of murin P2X4 (CT-X4) or a control peptide CT-3A (top) from spinal cord protein extracts of WT mice. After pull-down assay, eluted proteins were separated on a SDS-PAGE gel and revealed by western blot using anti-AP2 antibodies indicating that the C-tail of P2X4 interact specifically with AP2 while no signal was observed with CT-3A. E Pull-down assay using CT-X4 peptide of spinal proteins extracts from WT mice (P100) and SOD1 mice at P40, P75, P100 and P120 revealed using anti-AP2 antibodies as in D. Signals from inputs showed that AP2 expression is similar in all total protein extracts (see also Fig. S1 B–C). F The interaction between AP2 and P2X4 decreases over the time in SOD1 mice (significantly different from WT mice, in SOD1 mice at all ages (*p < 0.05, **p < 0.01, ***p < 0.001 one-way ANOVA)