Abstract
Background
Fatty infiltration of the paraspinal muscles may play a role in pain and disability in lumbar spinal stenosis. We assessed the reliability and association with clinical symptoms of a method for assessing fatty infiltration, a simplified muscle fat index (MFI).
Methods
Preoperative axial T2-weighted magnetic resonance imaging (MRI) scans of 243 patients aged 66.6 ± 8.5 years (mean ± standard deviation), 119 females (49%), with symptomatic lumbar spinal stenosis were assessed. Fatty infiltration was assessed using both the MFI and the Goutallier classification system (GCS). The MFI was calculated as the signal intensity of the psoas muscle divided by that of the multifidus and erector spinae. Observer reliability was assessed in 102 consecutive patients for three independent investigators by intraclass correlation coefficient (ICC) and 95% limits of agreement (LoA) for continuous variables and Gwet’s agreement coefficient (AC1) for categorical variables. Associations with patient-reported pain and disability were assessed using univariate and multivariate regression analyses.
Results
Interobserver reliability was good for the MFI (ICC 0.79) and fair for the GCS (AC1 0.33). Intraobserver reliability was good or excellent for the MFI (ICC range 0.86–0.91) and moderate to almost perfect for the GCS (AC1 range 0.55–0.92). Mean interobserver differences of MFI measurements ranged from -0.09 to -0.04 (LoA -0.32 to 0.18). Adjusted for potential confounders, none of the disability or pain parameters was significantly associated with MFI or GCS.
Conclusion
The proposed MFI demonstrated high observer reliability but was not associated with preoperative pain or disability.
Keywords: Magnetic resonance imaging, Paraspinal muscles, Patient-reported outcome measures, Psoas muscles, Spinal stenosis
Key points
Fatty infiltration of the paraspinal muscles can be quantified by magnetic resonance imaging (MRI).
The proposed muscle fat index (MFI) uses routine lumbar MRI examinations.
This novel MFI shows high observer reliability for the quantification of muscle fat.
Increased muscle fat was seen in patients with lumbar spinal stenosis (LSS).
Significant association between the MFI and symptoms of LSS was not found.
Background
Degenerative lumbar spinal stenosis (LSS) is a clinical condition caused by degenerative changes in the supporting structures of the lumbar spine [1]. Patients with LSS experience varying degrees of disability, low back pain, and radiating pain in lower extremities [2]. Fatty infiltration of the paraspinal muscles is a frequent finding in patients with LSS [3, 4]. Mainly formed by the multifidus (MF) and the erector spinae (ES), these muscles are innervated by the dorsal rami of the L1–L4 nerves. The main function of the paraspinal muscles is extension and rotation of the lumbar spine and to resist gravity [5]. Studies have demonstrated associations between the severity of fatty infiltration of the paraspinal muscles evaluated by magnetic resonance imaging (MRI), and pain and disability reported by patients with LSS [3, 6, 7]. It has been suggested that fatty infiltration of the paraspinal muscles can be used as a predictor of postoperative clinical outcomes and recovery of patients with symptomatic LSS, influencing the treatment decision process [8–10].
Imaging modalities can be used for the assessment and grading of the severity of fatty infiltration in the skeletal muscles. The Goutallier classification system (GCS) is a frequently used semiquantitative grading method for the assessment of muscle fatty infiltration [11]. This method was originally proposed by Goutallier et al. [12] for grading the severity of fatty infiltration in the shoulder rotator cuff muscles on computed tomography (CT) as a prognostic tool for tendon repairs, suggesting a poorer outcome when the cuff muscles had higher fatty infiltration. Fuchs et al. [13] demonstrated good or excellent interobserver reliability for the GCS on shoulder CT and MRI individually, but only fair to moderate correlation between the GCS grading performed on CT and MRI. Despite this inferior correlation, the GCS has been adopted for the evaluation of muscular fatty infiltration on MRI in various anatomical locations, including the paraspinal muscles [14–17]. Both quantitative and semiquantitative MRI methods have been used to assess the severity of fatty infiltration in the paraspinal muscles. It has been suggested that quantitative MRI methods have higher reliability than the semiquantitative methods [18–20]. The main drawbacks of the currently available quantitative methods are time consumption and the need for exporting the images into a third-party software for analysis, making these methods less practical in everyday clinical practice [7, 19, 21, 22].
The muscle fat index (MFI) is a quantitative measure used by researchers to assess the fat content of the paraspinal muscles on MRI, by calculating the ratio of the mean signal intensity of the muscle of interest to a homogenous area of the same or another muscle [23]. In the current study, we introduced a new method for calculating the MFI based on the signal intensity of the paraspinal and the psoas major (PM) muscles measured on axial T2-weighted images from routine lumbar spine MRI examinations, without a need for using a third-party software. To our knowledge, this simplified method for calculation of the MFI has not been used earlier. We hypothesised that this easily accessible method might yield higher reliability than the GCS and, furthermore, would associate with the clinical symptoms. The purpose of this study was to evaluate the reliability of this novel MFI and assess its association with pain and disability in patients with LSS.
Methods
Study participants
The regional committees for medical research ethics approved the current cross-sectional study (reference number: 2011/2034 central region). The study adhered to the Declaration of Helsinki and all patients provided written informed consent. The participants in this study were consecutively enrolled from the spinal stenosis trial of the Norwegian Spinal Stenosis and Degenerative Spondylolisthesis (NORDSTEN) study. This multicentre trial includes symptomatic patients with LSS without degenerative spondylolisthesis who are scheduled for surgery. The study protocol and the settings for inclusion and exclusion of the patients have been published earlier [24]. The inclusion and exclusion criteria for the current study are provided in Table 1. After the initial consecutive enrolment of 300 patients (convenient sampling based on the availability of patient data), we excluded 57 patients due to inadequate or missing images, leaving 243 patients who were finally included (Fig. 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
• Age between 18 and 80 years • Clinical symptoms of LSS • Not responding to at least 3 months of non-surgical treatment • Radiological findings (foraminal, central canal, or lateral recess stenosis) corresponding to the clinical symptoms such as back pain, leg pain, or neurologic claudication • Understanding the Norwegian language (spoken and written) |
• Previous surgery at the level of stenosis • Previous fracture or fusion of the thoraco-lumbar spine • Cauda equina syndrome (bowel or bladder dysfunction) or fixed complete motor deficit • ASA grade 4 or 5 • More than 20° lumbosacral scoliosis • Distinct symptoms in lower limbs due to other diseases • Stenosis in more than three lumbar levels • Being unable to comply fully with the protocol • Isthmic defect in pars interarticularis at the level of stenosis • Participation in another clinical study that could interfere with the present trial • Alcohol or substance abuse • ≥ 3 mm spondylolisthesis verified on upright lateral view x-ray • Axial T2-weighted MR images not covering the paraspinal and the psoas muscles at both sides of the spine or angulated more than 5° to the upper endplate of the vertebra at the level of measurement |
ASA American Society of Anesthesiologists, LSS Lumbar spinal stenosis
Fig. 1.

The flowchart shows the patient selection process. SST Spinal stenosis trial, NORDSTEN Norwegian degenerative spondylolisthesis and spinal stenosis
MRI protocol and assessments
The preoperative MRI examinations used in this study were performed at the local study sites of the NORDSTEN study between February 2013 and August 2016 using 1.5-T or 3.0-T units from several manufacturers, with patients in supine position. All images were anonymised and stored in a dedicated server. To maintain homogeneity of the examinations, the performing institutions were provided with a standardised MRI protocol including axial and sagittal T2-weighted and sagittal T1-weighted images. A board-certified radiologist (H.B.) verified whether the qualities of the images were adequate for the present study (e.g., the axial images covering both the paraspinal and the PM muscles on both sides of the spine). All measurements for the present study were performed on the axial T2-weighted images (repetition time 1,500–6,548 ms; echo time 82–126 ms; slice thickness 3–4 mm; field of view from 160 × 160 to 220 × 220 mm2).
The paraspinal (ES and MF) and the PM muscles were evaluated bilaterally at the level with the upper endplates of L3, L4, and L5 (for both quantitative assessments of the MFI and semiquantitative assessments of the GCS). Inspired by previous studies [21, 23], the investigators segmented the paraspinal and the psoas muscles by drawing manual regions of interest around each muscle group. All segmentations were done using the integrated measurement tools in a Picture Archiving and Communication System (PACS) (Sectra, Linkoping, Sweden) on personal laptops with non-diagnostic monitors. The mean signal intensity of the MF and the ES muscles was measured by drawing a region of interest around both muscles, excluding the epimuscular fat. The signal intensity of the muscles for each region of interest was calculated automatically by the PACS. To assess the relationship between the fatty infiltration of the paraspinal and the PM muscles, we used the PM muscle as a natural control. It has been suggested that the PM muscle is less prone to fatty infiltration [3, 7, 25]. The MFI was calculated as a continuous variable by dividing the mean signal intensity of the PM with the mean signal intensity of the MF and ES on the same image slice and side. In this way, values close to 1.0 indicated near equal proportions of fat and muscle fibres in the paraspinal muscles compared to the PM, suggesting a very low degree of fatty infiltration; values close to zero suggested a very high degree of fatty infiltration in the paraspinal muscles. An example of this measurement method is shown in Fig. 2.
Fig. 2.
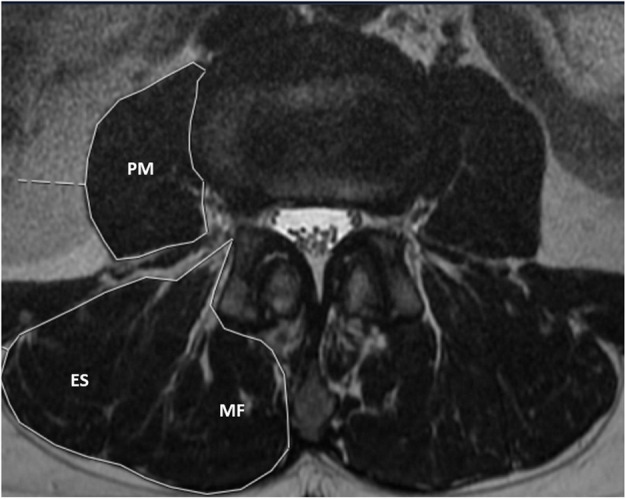
Axial T2-weighted magnetic resonance image obtained at the level of the upper endplate of L3. The muscle fat index (MFI) was calculated by dividing the mean signal intensity of the psoas major (PM) with the mean signal intensity of the erector spinae (ES) and the multifidus (MF) muscles
In the next stage (during the same session and on the same image slice used for calculation of the MFI), the severity of fatty infiltration was graded using the GCS as grade 0 (no fatty streaks), grade 1 (some fatty streaks), grade 2 (fatty infiltration but still more muscle fibres than fat), grade 3 (equal amounts of fat and muscle fibres), or grade 4 (larger amounts of fat than muscle fibres) [12].
Assessment of observer reliability
Interobserver and intraobserver reliability for both methods were assessed for measurements performed at the levels from L2 to L5 for the first 102 consecutive patients. The investigators were three independent observers who were blinded to each other’s measurements and to the severity of pain and disability of the patients. They were two orthopaedic spine surgeons (E.H. and J.A. with 10 and 6 years of experience, respectively) and a musculoskeletal radiologist (H.B. with 13 years of experience in spine imaging). To assess the intraobserver reliability and to maintain the independency of the test-retest readings, all observers repeated the evaluations after a minimum of 6 weeks, blinded to the results of their first readings. Images with missing measurements or non-optimal axial T2-weighted images (e.g., incomplete imaging of the muscles) were excluded and only levels with measurements from all the three observers were included in the reliability analyses. Prior to the study start, the investigators discussed the measurement criteria for both methods, and the segmentation method was presented to the orthopaedic spine surgeons by the radiologist. They performed test measurements of both the MFI and the GCS on 10 randomly chosen MRI examinations from the study population. The results of the test readings were not included in the statistical calculations.
Assessment of clinical symptoms
Patient-reported outcome measures were used for clinical assessment of pain and disability, including:
The Oswestry disability index (ODI) [26], a pain and disability index for use in low back pain ranging from 0 to 100, where 0 denotes no disability and 100 indicates complete disability
The Zurich claudication questionnaire (ZCQ) for pain and disability [27], a disease-specific questionnaire for LSS with several sub-scores including the severity of the symptoms and level of physical activity, ranging from 1 to 5, where 1 indicates the best clinical outcome
A numeric rating scale (NRS) for back and leg pain ranging from 0 to 10, where 0 indicates no pain and 10 indicates the worst pain imaginable [28]
Statistical analyses
Continuous variables were described as means ± standard deviations and categorical variables as frequencies and percentages. Intraclass correlation coefficient (ICC) was calculated using two-way random effects models for absolute agreement and was used to assess the interobserver and intraobserver reliability for the MFI. Bland-Altman plots were used to assess the mean differences and 95% limits of agreements (LoA) for repeated measurements. The categorical ratings of the GCS were unevenly distributed, and thus, we used Gwet’s agreement coefficient (AC1) instead of κ statistics to assess the interobserver and intraobserver agreements (to avoid the so-called high agreement low kappa paradox) [29]. 95% confidence intervals (CIs) were calculated for both ICC and AC1. ICC values were interpreted to indicate poor (< 0.50), moderate (0.51–0.75), good (0.76–0.90), and excellent (> 0.91) agreement [30] and AC1 values to indicate poor (0.0), slight (0.01–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect agreement (0.81–1.00) [31].
Observer 3 (radiologist H.B.) performed MFI measurements (continuous) and grading of the GCS (categorical) in the total study sample (243 patients). In few cases, the MFI values were higher than 1.0 and in the absence of apparent fatty infiltration in the PM, these values were redefined as 1.0. The measurements performed by observer 3 were used in the regression analyses and did not differ significantly between lumbar levels or sides (left/right). Thus, the values representing the highest fatty infiltration (lowest MFI or highest GCS values) from the L2/L3 level were entered into univariate and multivariate regression models, treating all the patient-reported outcome measures as continuous variables. Regression coefficients with corresponding 95% CIs were reported. In the multivariate regression models, we adjusted for age, sex, body mass index, and smoking status (yes or no). Because of the low prevalence of higher GCS grades and for better clinical relevance, we trichotomised the GCS values into category 0 (GCS grade 0, no fatty infiltration), category I (GCS grade 1, mild fatty infiltration), and category II (GCS grades 2 to 4, moderate or severe fatty infiltration) (Table 2). Model assumptions were assessed by normality plots of the standardised residuals and the fitted values. To compare the goodness of fit between the regression models, we calculated the Akaike information criterion (AIC). The AIC is a goodness of fit measure for comparing two models, where the regression model with the lowest AIC value fits better to the data. It has been suggested that an AIC difference of 2 to 7 should be considered as a meaningful difference between two models [32]; others have suggested a minimum difference of 6 AIC units [33]. Values of p lower than 0.05 were considered statistically significant. STATA software (StataCorp. LLC 2017. Stata Statistical Software: Release 16.1 College Station, TX, USA) was used for the statistical analyses.
Table 2.
Patient characteristics and distribution of MRI findings
| Patient characteristics (n = 243) | Mean ± standard deviation/count (%) |
|---|---|
| Age (years) | 66.6 ± 8.5 |
| Female | 119 (49) |
| BMI | 27.8 ± 4.0 |
| Smokers | 55 (23) |
| DSCA < 75 mm2 | |
|
L2/L3 L3/L4 L4/L5 |
31 (19) 106 (44) 157 (65) |
| ODI score (100-point scale) | 40.66 ± 14.57 |
| NRS back pain (10-point scale) | 6.28 ± 2.19 |
| NRS leg pain (10-point scale) | 6.38 ± 2.10 |
| ZCQ pain (5-point scale) | 3.38 ± 0.55 |
| ZCQ disability (5-point scale) | 2.58 ± 0.52 |
| MRI findings (L2/L3 level) | |
| MFI* | 0.53 ± 0.18 |
| GCS | |
|
Category 0 (grade 0) Category 1 (grade 1) Category 2 (grades 2, 3, and 4) |
119 (49) 100 (41) 24 (10) |
BMI Body mass index, DSCA Dural sac cross-sectional area, GCS Goutallier classification system, MFI Muscle fat index, NRS Numeric rating scale, ODI Oswestry disability index, ZCQ Zurich claudication score
*In one case, the value of the MFI was > 1.0, redefined as 1.0
Results
Patient characteristics
Patient characteristics and distribution of the MRI findings are presented in Table 2. The mean age was 66.6 years and 119 of the 243 included patients (49%) were women. The mean MFI value was 0.53, suggesting overall more than twice fat inside the paraspinal muscles compared to the PM. Most of the patients (n = 219, 90%) had GCS categories 0 or 1 (suggesting no or mild fatty infiltration in the paraspinal muscles) and 24/243 patients (10%) had moderate or severe fatty infiltration (categories 2 to 4). For the reliability part of the study (measurements at the L2–L5 levels), there were 424 GCS assessments and 418 MFI assessments per observer (not included in Table 2). There was an inverse relationship between the different grades of the GCS and the MFI values, indicating higher GCS grades in patients with lower MFI values (Table 3).
Table 3.
Relationship between the MFI and the GCS
| GCS grade | Mean MFI | 95% CI |
|---|---|---|
| 0 | 0.61 | 0.54, 0.68 |
| 1 | 0.42 | 0.39, 0.44 |
| 2 | 0.35 | 0.32, 0.37 |
| 3 | 0.30 | 0.26, 0.34 |
| 4 | 0.29 | 0.23, 0.35 |
CI Confidence interval, GCS Goutallier classification system, MFI Muscle fat index
Interobserver and intraobserver reliability
The results of the reliability analyses are presented in Table 4. The agreement coefficients suggested good overall interobserver agreement for the MFI and only fair agreement for the GCS. Intraobserver agreement for the three observers was good or excellent for the MFI, while for the GCS, the agreement values ranged from moderate to almost perfect.
Table 4.
Interobserver and intraobserver reliability
| Reliability | MFI | GCS |
|---|---|---|
| ICC (95% CI) | AC1 (95% CI) | |
| Interobserver | 0.79 (0.70, 0.85) | 0.33 (0.27, 0.39) |
| Intraobserver | ||
| Observer 1 | 0.91 (0.89, 0.92) | 0.92 (0.89, 0.95) |
| Observer 2 | 0.86 (0.74, 0.91) | 0.64 (0.59, 0.70) |
| Observer 3 | 0.91 (0.89, 0.93) | 0.55 (0.49, 0.60) |
AC1 Gwet’s agreement coefficient, CI Confidence interval, GCS Goutallier classification system, ICC Intraclass correlation coefficient, MFI Muscle fat index
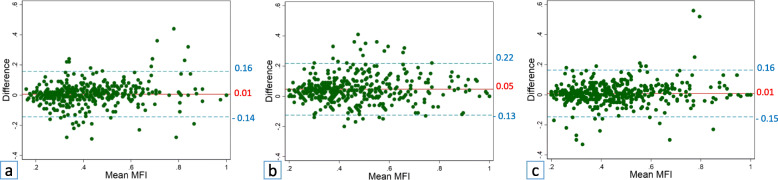
Measurement differences for the MFI between all observer pairs, as well as within the observers, are demonstrated by Bland-Altman plots in Figs. 3 and 4, respectively. Mean interobserver differences (i.e., mean bias) ranged from -0.09 to -0.04 with 95% LoA ranging from -0.32 to 0.18. The narrowest LoA for measurements (i.e., the smallest measurement differences) were observed between observers 2 and 3 (one of the two surgeons and the radiologist, Fig. 3c).
Fig. 3.
Bland-Altman plots with mean differences in measurements of the muscle fat index (MFI, solid lines) and 95% limits of agreement (dashed lines) between observers 1 and 2 (a), observers 1 and 3 (b), and observers 2 and 3 (c)
Fig. 4.
Bland-Altman plots with mean differences in measurements of the muscle fat index (MFI, solid lines) and 95% limits of agreement (dashed lines) for repeated measurements by observer 1 (a), observer 2 (b), and observer 3 (c)
Mean intraobserver differences ranged from 0.01 to 0.05 with 95% LoA ranging from -0.15 to 0.22. The narrowest LoA was achieved for observer 1 (one of the two surgeons, Fig. 4a).
Association with clinical symptoms
The results of the univariate regression analyses are presented in Table 5. The estimated regression coefficients were generally small. We found a significant association only between NRS leg pain and the MFI (p = 0.042). A tendency towards lower AIC values was observed for the MFI (suggesting a better fitting to the univariate regression models of the MFI compared to the GCS).
Table 5.
Univariate regression analyses
| Clinical parameter | MFI | GCS* | |||||
|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p | AIC | Category | Coefficient (95% CI) | p | AIC | |
| ODI | -6.90 (-17.38, 3.57) | 0.195 | 1940 | 1 | 2.65 (-3.91, 9.21) | 0.427 | 1945 |
| 2 | 10.28 (-6.40, 26.96) | 0.226 | |||||
| NRS leg pain | -1.58 (-3.11, -0.06) | 0.042 | 973 | 1 | 0.56 (-0.40, 1.52) | 0.249 | 978 |
| 2 | 0.66 (-1.72, 3.04) | 0.584 | |||||
| NRS back pain | -0.41 (-2.03, 1.21) | 0.618 | 1011 | 1 | 0.86 (-0.15, 1.87) | 0.093 | 1010 |
| 2 | 1.48 (-1.02, 3.97) | 0.245 | |||||
| ZCQ pain | -0.30 (-0.71, 0.10) | 0.136 | 388 | 1 | 0.06 (-0.20, 0.33) | 0.629 | 394 |
| 2 | 0.35 (-0.29, 0.99) | 0.281 | |||||
| ZCQ disability | -0.25 (-0.63, 0.13) | 0.191 | 368 | 1 | 0.08 (-0.16, 0.32) | 0.514 | 370 |
| 2 | 0.51 (-0.09, 1.11) | 0.098 | |||||
AIC Akaike information criterion, CI Confidence interval, GCS Goutallier classification system, MFI Muscle fat index, NRS Numeric rating scale, ODI Oswestry disability index, ZCQ Zurich claudication questionnaire
*For the GCS, only categories with fatty infiltration are presented. The coefficient values for the GCS show the estimates for the regression equation of categories 1 and 2, respectively, on category 0 (GCS grade 0, no fatty infiltration)
The results of the multivariate regression analyses are presented in Table 6. After adjusting for the potentially confounding factors, there were no significant associations between the patient-reported outcome measures and the MFI or the GCS. AIC values were consistently lower for the MFI and were 6 or 7 units lower in the analyses of the ODI and the ZCQ pain, suggesting better fitting of the MFI to the multivariate regression models.
Table 6.
Multivariate regression analyses
| Clinical parameter | MFI | GCS* | |||||
|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p | AIC | Category | Coefficient (95% CI) | p | AIC | |
| ODI | -3.74 (-14.69, 7.21) | 0.502 | 1863 | 1 | 0.10 (-5.69, 7.69) | 0.769 | 1869 |
| 2 | 7.06 (-0.26, 23.38) | 0.395 | |||||
| NRS leg pain | -0.59 (-2.13, 0.96) | 0.455 | 913 | 1 | 0.21 (-0.70, 1.12) | 0.649 | 916 |
| 2 | -0.23 (-244, 1.98) | 0.837 | |||||
| NRS back pain | 0.80 (-0.84, 2.44) | 0.339 | 950 | 1 | 0.63 (-0.34, 1.60) | 0.199 | 952 |
| 2 | 0.75 (-1.61, 3.10) | 0.533 | |||||
| ZCQ pain | -0.22 (-0.64, 0.20) | 0.205 | 364 | 1 | -0.01 (-0.26, 0.26) | 0.996 | 371 |
| 2 | 0.24 (-0.39, 0.86) | 0.454 | |||||
| ZCQ disability | -0.01 (-0.30, 0.03) | 0.979 | 344 | 1 | 0.01 (-0.24, 0.25) | 0.972 | 347 |
| 2 | 0.40 (-0.19, 0.99) | 0.186 | |||||
AIC Akaike information criterion, CI Confidence interval, GCS Goutallier classification system, MFI Muscle fat index, NRS Numeric rating scale, ODI Oswestry disability index, ZCQ Zurich claudication questionnaire
*For the GCS, only categories with fatty infiltration are presented. The coefficient values for the GCS show the estimates for the regression equation of categories 1 and 2, respectively, on category 0 (GCS grade 0, no fatty infiltration)
Discussion
In this study, we found a high observer reliability for a novel quantitative MRI method (simplified MFI) in the assessment of fatty infiltration in the paraspinal muscles of patients with symptomatic LSS. For a more established semiquantitative method (the GCS), interobserver reliability was only fair and intraobserver reliability ranged from moderate to almost perfect. We found a significant association between leg pain and the MFI in the univariate regression analyses, but no significant associations in the multivariate analyses. However, the reliability coefficients and the AIC values suggested that the MFI presented here is a better fit to the regression models than the GCS.
Other quantitative methods have been used to assess the fatty infiltration of the paraspinal muscles on MRI. Researchers have used different software applications for texture analysis of the paraspinal muscles [22] or to assess the lean mass of the muscles by thresholding the signal intensity on MR images [7]. Both texture analysis and thresholding of the paraspinal muscles have shown high reliability [19, 21]. DIXON methods have gained increasing interest in spine imaging [34] and have been used for the quantification of fatty infiltration of the paraspinal muscles [35]. However, the need for exporting imaging data into a third-party software and performing additional MRI sequences makes these methods less practical in everyday practice. Whether the proposed MFI in the current study can be used on axial DIXON images of the lumbar spine needs further investigation.
Previous research has mainly focussed on the associations between back pain and fatty infiltration [6, 8, 22, 36–38]. Leg pain is a frequent symptom in patients with LSS and it is generally accepted that this symptom is a radiating pain from the lumbar spine [2]. There are, however, some controversies on whether this pain is solely generated by the lumbar nerves or can partly be caused by supporting structures of the spine [39, 40]. It has been suggested that patients with LSS and predominant leg pain are more likely to benefit from surgical decompression compared to those with predominant back pain [41]. We did not find a significant association between fatty infiltration of the paraspinal muscles and leg pain after adjusting for potential confounding factors. To our knowledge, this is the first study to examine this association in patients with LSS. Two studies not concerning LSS assessed leg pain in relation to fatty infiltration of the MF muscle with conflicting results. Fatty infiltration of the MF muscle was associated with leg pain in a retrospective study of 78 patients with low back pain [42] but not in a population-based cohort study of young individuals with a history of leg or back pain [43].
Several studies have examined the relationship between fatty infiltration of the paraspinal muscles and symptoms of degenerative diseases of the lumbar spine [6, 15, 23, 44, 45], but not all studies have considered the role of the PM muscle in this relationship [6, 15, 45]. To estimate the degree of fatty imbalance between the PM and the paraspinal muscles, we calculated the MFI by dividing the signal intensity of the PM with that of the MF and ES. The calculated mean MFI of 0.53 in the current study suggested overall less fatty infiltration in the PM compared to the MF and ES muscles, which is in accordance with previous research [3, 7, 25]. The important role of denervation in atrophy and fatty infiltration of the skeletal muscles have been demonstrated [46–48]. Higher fatty infiltration in the paraspinal muscles compared to the PM may support the role of damage of the dorsal rami of the lumbar nerves as a cause of fatty infiltration [49–51]. It is unclear whether nerve damage can be a common cause for fatty infiltration of the paraspinal muscles and leg pain in patients with LSS. However, it is important to be aware of this possible association in clinical practice. The MFI presented in this study provides a reliable and easy-to-perform quantitative method for assessment of fatty infiltration in the paraspinal muscles on a standard clinical MRI examination without a need of additional software resources and with a high potential to widespread use.
A limitation of this study was the highly symptomatic surgical sample, potentially leading to an underestimation of any association between fatty infiltration and symptoms (due to potential restriction of range) [52]. Furthermore, the results of this study are limited to patients with LSS. Another limitation that may have influenced the reliability was the heterogeneity of the MR images. Images obtained from different MRI units and manufacturers can differ in brightness, affecting the perception of the signal intensity. This may partly explain the lower reliability for the subjectively evaluated GCS in this study, but hardly affected the MFI measurements. We excluded the epimuscular fat of the paraspinal muscles in the MFI measurements; some studies have included this fat in quantitative measurements. There is, however, a lack of consensus on whether the epimuscular fat should be included or excluded from the measurements [20].
We did not measure time consumption in this study, but time is an important factor in clinical and radiological everyday practice. Quantitative MRI methods are generally more time-consuming compared to semiquantitative and qualitative methods [20]. The advent of artificial intelligence methods for automated segmentation of muscles and the integration of these methods with clinical PACS solutions are expected to resolve the time-consumption issue [53]. We used the signal intensity of the muscles for the assessment of fatty infiltration. It can be argued that the proportion of fat and muscle fibres (used in the GCS) can be applied in artificial intelligence methods to improve the assessment of fatty infiltration in the paraspinal muscles as well (e.g., by calculating the lean muscle to fat ratio). Whether such method would result in better reliability and association with the clinical symptoms of patients with LSS is yet to be examined.
This simplified MFI method using routine MR images should be investigated in a broader patient population with LSS, also including patients without the need of surgical treatment, as well as to see whether fatty infiltration of the paraspinal muscles can be used as a predictor for postoperative outcomes of LSS.
In conclusion, the novel MFI proposed in this study presents a highly reliable method for the assessment of fatty infiltration in the paraspinal muscles using routine spine MRI examinations and measurement tools available in the PACS solutions. This MFI was not significantly associated with pain and disability in LSS but may provide better explanation for symptoms related to fatty infiltration in the paraspinal muscles, compared to the GCS.
Acknowledgements
We thank the members of the NORDSTEN working group, the staff at the Research and Communication Unit for Musculoskeletal Health (FORMI) at Oslo University Hospital, and the representative for the Norwegian Spine Association Inger Ljostad.
Abbreviations
- AIC
Akaike information criterion
- CI
Confidence interval
- CT
Computed tomography
- ES
Erector spinae
- GCS
Goutallier classification system
- ICC
Intraclass correlation coefficient
- LoA
Limits of agreements
- LSS
Lumbar spinal stenosis
- MF
Multifidus
- MFI
Muscle fat index
- MRI
Magnetic resonance imaging
- NRS
Numeric rating scale
- ODI
Oswestry disability index
- PACS
Picture Archiving and Communication System
- PM
Psoas major
- ZCQ
Zurich claudication questionnaire
Authors’ contributions
HB, EH, JA, AN, MA, KS, MG, AE, KI, and CH have designed the current study and have been involved in the acquisition and interpretation of data. HB, TÅM, AE, MG, AN, JIB, CW, and HeB have evaluated the methods and statistics. HB is the primary investigator for this study and has drafted the manuscript under the supervision of EH and AN. All the listed authors have critically revised the manuscript and approved the final version.
Funding
This work was supported by the Liaison Committee for Education, Research and Innovation in Central Norway (grant number: 2013/10174) and Sophies Minde Foundation in Norway (grant number: 02/2019). The funding sources did not have any involvement in the study design, collection, analysis, and interpretation of data and writing or decision to submit the article for publication.
Availability of data and materials
The datasets produced during this study are available from the corresponding author upon a reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Norwegian regional committees for medical research ethics (Reference number: 2011/2034 Central region). All patients signed written informed consent.
Consent for publication
Not applicable
Competing interests
None
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24:253–265. doi: 10.1016/j.berh.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358:818–825. doi: 10.1056/NEJMcp0708097. [DOI] [PubMed] [Google Scholar]
- 3.Chen YY, Pao JL, Liaw CK, Hsu WL, Yang RS. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur Spine J. 2014;23:999–1006. doi: 10.1007/s00586-013-3148-z. [DOI] [PubMed] [Google Scholar]
- 4.Yarjanian JA, Fetzer A, Yamakawa KS, Tong HC, Smuck M, Haig A. Correlation of paraspinal atrophy and denervation in back pain and spinal stenosis relative to asymptomatic controls. PM R. 2013;5:39–44. doi: 10.1016/j.pmrj.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Kalimo H, Rantanen J, Viljanen T, Einola S. Lumbar muscles: structure and function. Ann Med. 1989;21:353–359. doi: 10.3109/07853898909149220. [DOI] [PubMed] [Google Scholar]
- 6.Cooley JR, Walker BF, Ardakani EM, Kjaer P, Jensen TS, Hebert JJ (2018) Relationships between paraspinal muscle morphology and neurocompressive conditions of the lumbar spine: a systematic review with meta-analysis. BMC Musculoskelet Disord 19:351. 10.1186/s1289-018-2266-5 [DOI] [PMC free article] [PubMed]
- 7.Fortin M, Lazáry À, Varga PP, Battié MC. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. 2017;26:2543–2551. doi: 10.1007/s00586-017-5228-y. [DOI] [PubMed] [Google Scholar]
- 8.He K, Head J, Mouchtouris N, et al. The implications of paraspinal muscle atrophy in low back pain, thoracolumbar pathology, and clinical outcomes after spine surgery: a review of the literature. Global Spine J. 2020;10:657–666. doi: 10.1177/2192568219879087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori Y, Hoshino M, Inage K, et al. Clinical importance of trunk muscle mass for low back pain, spinal balance, and quality of life—a multicenter cross-sectional study. Eur Spine J. 2019;28:914–921. doi: 10.1007/s00586-019-05904-7. [DOI] [PubMed] [Google Scholar]
- 10.Storheim K, Berg L, Hellum C, et al. Fat in the lumbar multifidus muscles - predictive value and change following disc prosthesis surgery and multidisciplinary rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up of a randomized trial. BMC Musculoskelet Disord. 2017;18:145. doi: 10.1186/s12891-017-1505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somerson JS, Hsu JE, Gorbaty JD, Gee AO. Classifications in Brief: Goutallier classification of fatty infiltration of the rotator cuff musculature. Clin Orthop Relat Res. 2016;474:1328–1332. doi: 10.1007/s11999-015-4630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. doi: 10.1097/00003086-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 14.Battaglia PJ, Maeda Y, Welk A, Hough B, Kettner N. Reliability of the Goutallier classification in quantifying muscle fatty degeneration in the lumbar multifidus using magnetic resonance imaging. J Manipulative Physiol Ther. 2014;37:190–197. doi: 10.1016/j.jmpt.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Mandelli F, Nüesch C, Zhang Y, et al. Assessing fatty infiltration of paraspinal muscles in patients with lumbar spinal stenosis: Goutallier classification and quantitative MRI measurements. Front Neurol. 2021;12:656487. doi: 10.3389/fneur.2021.656487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemt C, Simeone FJ, Melnic CM, Tirumala V, Xiong L, Kwon YM. MARS MRI assessment of fatty degeneration of the gluteal muscles in patients with THA: reliability and accuracy of commonly used classification systems. Skeletal Radiol. 2021;50:665–672. doi: 10.1007/s00256-020-03611-9. [DOI] [PubMed] [Google Scholar]
- 17.Thompson SM, Reilly P, Emery RJ, Bull AM. A comparison of the degree of retraction of full-thickness supraspinatus tears with the Goutallier grading system. J Shoulder Elbow Surg. 2012;21:749–753. doi: 10.1016/j.jse.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Han G, Jiang Y, Zhang B, Gong C, Li W. Imaging evaluation of fat infiltration in paraspinal muscles on MRI: a systematic review with a focus on methodology. Orthop Surg. 2021;13:1141–1148. doi: 10.1111/os.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannil M, Burgstaller JM, Thanabalasingam A, et al. Texture analysis of paraspinal musculature in MRI of the lumbar spine: analysis of the lumbar stenosis outcome study (LSOS) data. Skeletal Radiol. 2018;47:947–954. doi: 10.1007/s00256-018-2919-3. [DOI] [PubMed] [Google Scholar]
- 20.Hodges PW, Bailey JF, Fortin M, Battié MC. Paraspinal muscle imaging measurements for common spinal disorders: review and consensus-based recommendations from the ISSLS degenerative spinal phenotypes group. Eur Spine J. 2021;30:3428–3441. doi: 10.1007/s00586-021-06990-2. [DOI] [PubMed] [Google Scholar]
- 21.Fortin M, Battié MC. Quantitative paraspinal muscle measurements: inter-software reliability and agreement using OsiriX and ImageJ. Phys Ther. 2012;92:853–864. doi: 10.2522/ptj.20110380. [DOI] [PubMed] [Google Scholar]
- 22.Mannil M, Burgstaller JM, Held U, Farshad M, Guggenberger R. Correlation of texture analysis of paraspinal musculature on MRI with different clinical endpoints: Lumbar Stenosis Outcome Study (LSOS) Eur Radiol. 2019;29:22–30. doi: 10.1007/s00330-018-5552-6. [DOI] [PubMed] [Google Scholar]
- 23.D'Hooge R, Cagnie B, Crombez G, Vanderstraeten G, Dolphens M, Danneels L. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Manual Therapy. 2012;17:584–588. doi: 10.1016/j.math.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Hermansen E, Austevoll IM, Romild UK, et al. Study-protocol for a randomized controlled trial comparing clinical and radiological results after three different posterior decompression techniques for lumbar spinal stenosis: the Spinal Stenosis Trial (SST) (part of the NORDSTEN Study) BMC Musculoskelet Disord. 2017;18:121. doi: 10.1186/s12891-017-1491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbanas J, Pavlovic I, Marijancic V, et al. MRI features of the psoas major muscle in patients with low back pain. Eur Spine J. 2013;22:1965–1971. doi: 10.1007/s00586-013-2749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976) 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 27.Stucki G, Daltroy L, Liang MH, Lipson SJ, Fossel AH, Katz JN. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine (Phila Pa 1976) 1996;21:796–803. doi: 10.1097/00007632-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29–48. doi: 10.1348/000711006x126600. [DOI] [PubMed] [Google Scholar]
- 30.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropractic medicine. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 32.Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol. 2011;65:23–35. doi: 10.1007/s00265-010-1029-6. [DOI] [Google Scholar]
- 33.Stylianou C, Pickles A, Roberts SA. Using Bonferroni, BIC and AIC to assess evidence for alternative biological pathways: covariate selection for the multilevel Embryo-Uterus model. BMC Med Res Methodol. 2013;13:73–73. doi: 10.1186/1471-2288-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanchi F, Richard R, Hussami M, Monier A, Knebel JF, Omoumi P. MRI of non-specific low back pain and/or lumbar radiculopathy: do we need T1 when using a sagittal T2-weighted Dixon sequence? Eur Radiol. 2020;30:2583–2593. doi: 10.1007/s00330-019-06626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Huang M, Serrano Sosa M, et al. Fatty infiltration of paraspinal muscles is associated with bone mineral density of the lumbar spine. Arch Osteoporos. 2019;14:99. doi: 10.1007/s11657-019-0639-5. [DOI] [PubMed] [Google Scholar]
- 36.Steffens D, Hancock MJ, Maher CG, Williams C, Jensen TS, Latimer J. Does magnetic resonance imaging predict future low back pain? A systematic review. Eur J Pain. 2014;18:755–765. doi: 10.1002/j.1532-2149.2013.00427.x. [DOI] [PubMed] [Google Scholar]
- 37.Endean A, Palmer KT, Coggon D (2011) Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: a systematic review. Spine (Phila Pa 1976)36:160–169. 10.1097/BRS.0b013e3181cd9adb [DOI] [PMC free article] [PubMed]
- 38.Ranger TA, Cicuttini FM, Jensen TS, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017;17:1729–1748. doi: 10.1016/j.spinee.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Kellgren JH. Referred pains from muscle. Br Med J. 1938;1:325–327. doi: 10.1136/bmj.1.4023.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinclair DC, Feindel WH, et al. The intervertebral ligaments as a source of segmental pain. J Bone Joint Surg Br. 1948;30b:515–521. doi: 10.1302/0301-620X.30B3.515. [DOI] [PubMed] [Google Scholar]
- 41.Pearson A, Blood E, Lurie J, et al. Predominant leg pain is associated with better surgical outcomes in degenerative spondylolisthesis and spinal stenosis: results from the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2011;36:219–229. doi: 10.1097/BRS.0b013e3181d77c21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145–149. doi: 10.1053/crad.1999.0340. [DOI] [PubMed] [Google Scholar]
- 43.Hebert JJ, Kjaer P, Fritz JM, Walker BF. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine (Phila Pa 1976) 2014;39:1417–1425. doi: 10.1097/brs.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 44.Fortin M, Gibbons LE, Videman T, Battié MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand J Med Sci Sports. 2015;25:880–887. doi: 10.1111/sms.12301. [DOI] [PubMed] [Google Scholar]
- 45.Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2–2. doi: 10.1186/1741-7015-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haig AJ. Paraspinal denervation and the spinal degenerative cascade. Spine J. 2002;2:372–380. doi: 10.1016/s1529-9430(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 47.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976) 2006;31:2926–2933. doi: 10.1097/01.brs.0000248453.51165.0b. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012;94:e41. doi: 10.2106/jbjs.K.00620. [DOI] [PubMed] [Google Scholar]
- 49.Chon J, Kim H-S, Lee JH, et al. Asymmetric atrophy of paraspinal muscles in patients with chronic unilateral lumbar radiculopathy. Ann Rehabil Med. 2017;41:801–807. doi: 10.5535/arm.2017.41.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun D, Liu P, Cheng J, Ma Z, Liu J, Qin T. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC Musculoskelet Disord. 2017;18:167–167. doi: 10.1186/s12891-017-1522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rantanen J, Hurme M, Falck B et al (1993) The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine (Phila Pa 1976)18:568–574. 10.1097/00007632-199304000-00008 [DOI] [PubMed]
- 52.Warne RT. Statistics for the social sciences: a general linear model approach. Cambridge: Cambridge University Press; 2017. pp. 383–390. [Google Scholar]
- 53.Osorno-Castillo K, Fonnegra RD, Díaz GM. Integration of machine learning models in pacs systems to support diagnostic in radiology services. In: Figueroa-García JC, Garay-Rairán FS, Hernández-Pérez GJ, Díaz-Gutierrez Y, editors. Applied computer sciences in engineering. Cham: Springer International Publishing; 2020. pp. 233–244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets produced during this study are available from the corresponding author upon a reasonable request.