Background
In 1966, an article reported that the source of chronic low back pain (CLBP) could not be identified in 79% of male and 89% of female patients from a general practice population [1]. In the decades that followed many clinicians and researchers repeated this claim. Notably, the article’s authors had no specialized training or education in diagnosing or treating painful spine disorders; and their study was conducted prior to the advent of advanced imaging such as magnetic resonance imaging (MRI) and even computerized topography (CT). Conclusions that seemed founded at that time are no longer accurate, as knowledge of spine biochemistry, biomechanics, and pathophysiology evolved to allow a more sophisticated approach to the diagnoses and treatment of CLBP.
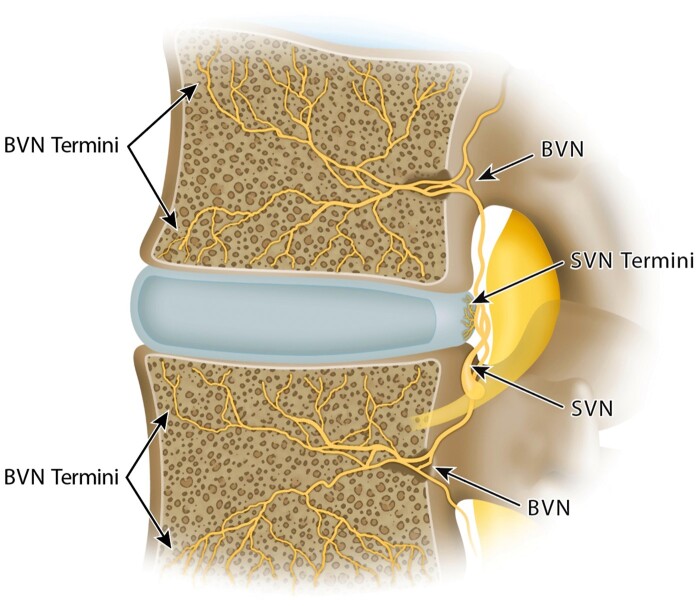
Chronic low back pain is a common symptom of a heterogeneous group of causative conditions. Clinicians and researchers have long recognized that better subgrouping of individuals with CLBP is necessary for more targeted and effective treatments. Commonly described sources of CLBP include the zygapophyseal joints, sacroiliac joints, and intervertebral discs (often termed “discogenic” pain) [2]. Historically, the term “discogenic pain” has been associated with disc degeneration and internal disc disruption with the presence of fissures in the annulus fibrosus and associated nociception via branches of the sinuvertebral nerve [3–6]. Previously, it was thought that pathological neurovascular ingrowth penetrated into annular fissures, leading to increased sensitivity and nociception via the sinuvertebral nerve [7, 8]. However, more recent evidence appears to refute the occurrence of such neurovascular ingrowth in many cases [9]. In the late 1990s, a team of researchers led by Dr. Heggeness reported that vertebral bodies were richly vascularized by vertebral capillaries and innervated by nociceptors that traced back to a single source, the basivertebral nerve [10]. Subsequently, it was demonstrated that the BVN is a branch of the sinuvertebral nerve (SVN) that enters the vertebral body through the foramen in its posterior wall, then it arborizes caudal and cephalad to densely innervate the vertebral endplates [11] (Figure 1). With progressive segmental degeneration or acute injury, altered force transfer and endplate stress can result in changes to endplate morphology and composition with concomitant impairment in permeability and transport [12, 13]. Histomorphology of human vertebral bodies demonstrated endplate nociceptor densification in areas of damage that were associated with increased disc degeneration. In addition, they found that only 30% of annular tears in degenerated discs had pathologic neural ingrowth, compared with 90% of adjacent endplates (which were twice as densely innervated) [9]. This distinction between annular and endplate innervation is likely due to differences in nerve ingrowth potential. For the annulus, nerve ingrowth is inhibited by physical pressure and proteoglycans [14, 15] and thus confined to proteoglycan-depleted annular fissures [16], whereas nerves can easily proliferate in fibrovascular bone marrow adjacent to sites of endplate damage [17]. Immunohistochemical studies of the BVN have demonstrated immunoreactivity to S-100, substance-P, and PGP 9.5 further supporting the BVN’s role in nociceptive innervation [10, 11, 17, 18]. Accumulated damage to the discovertebral complex may result in chemical and mechanical sensitization of endplate nocioceptors [17, 19]. These histopathological findings led to exploration of an “endplate-driven” model of discovertebral pain, with nociception largely occurring via the basivertebral nerve to a greater extent than the sinuvertebral nerve [10, 20–22]. This research supports an “endplate-driven” model of anterior column degeneration and existence of a fourth distinct structural source of low back pain, popularly termed vertebrogenic pain [23].
Figure 1.
Neuroanatomy of the lumbar discovertebral complex. SVN = sinuvertebral nerve; BVN = basivertebral nerve.
Although the vertebral body endplates categorically possess the prerequisite features necessary to cause CLBP, the clinical and radiographic characteristics which distinguish vertebrogenic pain (transmitted via the basivertebral nerve) from discogenic pain (annulus fibrosis pain transmitted via the sinuvertebral nerve) and other sources of CLBP were poorly characterized previously. The following article describes advances in our understanding of the phenomenon of “vertebrogenic pain” and how new clinical science, based on the described neuroanatomical and histopathological findings, is changing the diagnostic and therapeutic paradigm of axial CLBP.
Clinical Presentation of Vertebrogenic Low Back Pain
To date, few studies have been published which describe the clinical presentation of vertebrogenic low back pain (LBP). In studies that use a treatment response to BVN RFA as a proxy for a “true” diagnosis of vertebrogenic pain (as a superior reference standard has yet to be established), vertebrogenic pain at the L3–S1 levels appears to present as mid-line low lumbar pain with minimal cephalad referral but potentially some referral to the paraspinal and/or gluteal regions [24]. This is in contrast to the lumbosacral facet joints or the sacroiliac joint (SIJ) complex, which typically present with lateralized paraspinal (facet) and posterior superior iliac spines (PSIS) region pain (SIJ complex), respectively [25, 26]. Lumbosacral facet joint pain is typically associated with paraspinal tenderness on examination [27]. SIJ pain is associated with a number of provocation maneuvers that produce sheer, rotational, and/or compressive forces on the SIJ [28]. The SIJ complex may also be associated with thigh pain depending on age [2, 25, 26, 29]. Analysis of pain patterns of those successfully treated with BVN RFA showed that no patients reported pain below the knee, suggesting that pain referral from endplates does not produce more distal radiation [24]. However, this pattern could also be artifactual since many candidates for BVN RFA might have been excluded if they had significant symptoms concerning for radicular pain. It is possible that patients with vertebrogenic pain experience referral into the lower leg, but this should only be entertained after exclusion of other potential sources such as lumbar spinal stenosis (LSS), lumbar radiculopathy, nerve entrapment syndromes, peripheral neuropathy, or non-musculoskeletal causes of lower leg pain. To be clear, this pain pattern mirrors what was previously described as axial “discogenic” back pain. Compared to CLBP controls, patients with CLBP and Modic type 1 changes (MC1) are more likely to report night pain, prolonged morning stiffness, and pain greatest in the morning [30]. Patients with presumed vertebrogenic LBP frequently report pain exacerbation with activity and absence of pain exacerbation during lumbar extension.
Although some patients experience relatively “pure” vertebrogenic pain due to pathologic degeneration of the discovertebral complex, others may experience pain transmitted via the BVN with concomitant pain related to annular pain transmitted via the sinuvertebral nerve, as well as pain from compression of neural elements within the spinal canal and/or neuroforamen. Coexisting facet joint-related pain, with nociception via the medial branch nerves is also possible, particularly given the frequency of disc height loss in patients with vertebrogenic pain, which results in greater facet joint loading at the spinal motion segment [31]. Indeed, it has been reported that Modic type 1 changes (MC1) and endplate defects commonly co-occur in patients with lumbar disc herniation and associated radiculopathy [32, 33], and these findings are associated with higher rates of conservative treatment failure prior to discectomy [34]. In patients with LSS, endplate defects are a stronger predictor of axial LBP intensity than the severity of the spinal stenosis [35]. However, multiple peer reviewed research papers have reported a low prevalence of multifactorial LBP [2, 36]. Nonetheless, it appears that the presence or absence of midline LBP can help differentiate between joint pain and anterior column component pain and can assist the evaluating clinician when examining LBP patients [24, 26, 29].
Imaging Characteristics of Vertebrogenic Pain: Endplate Defects and Modic Changes
A correlation between vertebral endplate pathology on MRI and LBP was first suggested in 1988 by Modic et al. who found intraosseous MRI changes adjacent to vertebral endplates defects in individuals with chronic LBP [37]. Inflammation and bone marrow changes surrounding endplate defects are visible as Modic changes (MC) on MRI [38]. Type 1 Modic changes are associated with bone marrow edema and hypervascularity of the vertebral body displayed as decreased signal intensity on T1-weighted images, and increased signal intensity on T2-weighted images. Type 2 Modic changes (MC2) are associated with fatty replacement of the red bone marrow in the vertebral body and display as increased signal intensity on T1-weighted images and on T2-weighted images.
An association has been established between the presence of MC1 and MC2 of the vertebral endplates and disabling CLBP [39]. Histopathologic studies corroborate the existence of both granulation and fibrotic tissue in areas of MC1 and MC2, indicating that MC are likely a consequence of cycles of inflammation and healing. Weishaupt et al. reported 88–100% specificity to CLBP in individuals depending on the extent of MC1 and MC2, and Kuisma et al. found a 2.28 odds ratio for the presence of Modic changes at L5-S1 in individuals with CLBP [40, 41]. Although MC lesions may be observed in up to 12.1% of asymptomatic individuals, there remains an overall strong association between their presence and CLBP [42]. This association is particularly strong for MC1; a meta-analysis found an odds ratio of 4.01 for the presence of MC1 and CLBP, while only 3% of asymptomatic subjects exhibited MC1 [42].
In addition to MC, other endplate defects observed on MRI also strongly correlate with CLBP. Either acute injury or chronic repetitive injury to the endplate is likely the inciting event that permits leakage of disc secreted factors into the vertebral body bone marrow, leading to inflammation and/or fatty infiltration, fibrosis, and increased bone turnover that may subsequently be visualized as MC1 and/or MC2 [8]. In this model, MC can be thought of as a “late stage” finding, whereas endplate injury(ies) may be considered the sentinel event(s). A systematic review including over 11,000 subjects concluded that “erosive” type endplate defects are strongly associated with low back pain (odds ratio [OR] 2.69) [43] and larger endplate defects have been associated with greater LBP related disability in some studies. However, in analysis of data from BVN RFA trials, endplate defect presence, size, and morphology were not found to influence treatment success or failure, possibly because all patients enrolled in these trials had already had MC1 or MC2 (“late stage” disease) [44]. Interestingly, patients with smaller volume MC, non-centrally located MC, and those with <25% involvement of the endplate respond similarly as patients with large volume MC. Patients with MC1 vs MC2 experienced similar rates of success [44].
Spine specialists and musculoskeletal radiologists frequently encounter “degenerative spine syndrome” on MRI, where multilevel degeneration of the anterior and posterior columns coexist. Several studies examining how various degenerative findings impact the success of facet denervation procedures yielded mixed findings [45–49]. Until recently, there had been no description of how such findings impact treatment success of BVN RFA. Pooled analysis of multicenter trial data found that treatment success was not significantly impacted by degree of disc degeneration (Pfirrmann Grade), presence of high intensity zones, grade of facet joint arthropathy, or degree of foraminal, central canal, or lateral recess narrowing. The presence of facet joint fluid was associated with a lower probability of treatment success after BVN RFA (OR 0.578) but was considered a weak predictor of treatment outcome in the statistical model (area under the curve [AUC] 0.5609). It should be acknowledged that these observations were derived from strictly selected trial populations which excluded patients with clinical evidence of symptomatic spinal stenosis, radicular pain, lumbar facet joint pain or radiographic evidence of spondylolisthesis greater than 2 millimeters. In the presence of spondylolisthesis, facet joint effusions are strongly correlated with dynamic instability [50], however, population based cohort studies have shown that facet effusions alone are not significantly associated with the presence of LBP [51]. Considering this evidence, prior to considering BVN RFA, clinicians are encouraged to obtain standing flexion/extension lumbar radiographs in patients when there is clinical suspicion of segmental instability. Ruling out facet joint-mediated pain using medial branch nerve blocks (MBBs) should be considered when there is suspicion for facet joint pain, regardless of the presence of facet joint effusions [27].
Interventional Diagnosis of Vertebrogenic Low Back Pain
Given the complex and overlapping pain patterns of various structures within the lumbosacral region, clinicians have often utilized diagnostic/prognostic injections to test hypotheses formulated based upon the history, physical exam, and imaging. Examples of this include intra-articular SIJ anesthetic blocks, discography, and MBBs. While anesthetic administration to the proximal intraosseous portion of the BVN may initially appear attractive as a diagnostic test for vertebrogenic pain, there are several practical problems with this approach. Access without penetrating the vertebral bone would require an unacceptable transthecal needle path. As a result transpediclar access is required, which generally mandates conscious sedation and abundant opportunities for false positive or negative results. To access the pedicle, the territory of the traversing lumbar medial branch nerve would be encountered and anesthetized, which will further confuse assessment of the underlying source of pain. As a practical matter, additional invasive testing seems unjustified given the high rates of success and positive outcomes observed with BVN RFA when selection is based on clinical and radiographic criteria alone [52].
Lessons learned from decades of study related to spinal facet denervation procedures might also be applied to vertebrogenic pain. Although not a part of most standard practices, placebo-controlled triple blocks have been found to select patients with relatively “pure” zygapophyseal joint pain in the cervical and lumbar spine [53–55]. Patients selected by this type of paradigm are more likely to benefit from medial branch nerve RFA (compared to uncontrolled anesthetic blocks, which have a high false positive rate), but some have suggested that the practice of applying multiple blocks is expensive, and increases the rates of false negative responses, thus withholding treatment from some who could benefit [27, 56]. Even if a test to directly anesthetize the BVN were feasible, the costs and risks of such a practice would likely not be justified given that the current selection paradigm for BVN RFA results high rates of successful pain reduction, functional improvement, and reduction in healthcare utilization for 5 years or longer [57].
Provocation discography, while historically considered the gold standard for diagnosing “discogenic” pain [58], is of unclear value in differentiating pain arising from the disc annulus fibrosis vs the vertebral endplate. Although the probability of reproducing patient symptoms during provocation discography is significantly greater in patients with endplate damage [59], disc pressurization can potentially provoke nociception transmitted by both the annulus and the sinuvertebral nerve as well as the endplate and the basivertebral nerve, as pressurization results in stretch of annulus fibrosis fibers as well as endplate deflection [60, 61]. Endplate deflection of <1 mm occurs upon reaching intradiscal pressure of 75–100 psi in the absence of annular fissures [60, 62], but this deflection may be increased in the presence of endplate microdamage [63]. By definition, discs with internal derangement responsible for clinically meaningful CLBP produce such pain at <50 psi [59]. However, it is unknown how much endplate deflection can be evoked at low pressures in the presence of significant endplate damage. Furthermore, it is not known how much endplate deflection is necessary to provoke symptoms. It is sensible to presume that inflamed nerve endings in endplate are hypersensitive to any mechanical perturbation. The role of PLD to differentiate between painful annular fissures and painful VBE needs further study. Because of this, the role of provocation discography in identifying patients with vertebrogenic pain has yet to be clarified. Positive and negative responses to intradiscal anesthetic injection (i.e., functional anesthetic discography (FAD) or “discoblock”) are strongly correlated with the respective presence and absence of MC1 and MC2 [64, 65]. However, additional studies are needed to define the diagnostic characteristics of anesthetic discography in confirming or refuting vertebrogenic pain.
The diagnosis of vertebrogenic LBP should be strongly suspected when MRI demonstrates the presence of Modic 1 or 2 changes, with or without endplate defects. We encourage clinicians to apply evidenced based diagnostic tools to evaluate for alternative or comorbid spinal pain generators when suspected; this may include lumbar MBBs and/or intra-articular SIJ injections to evaluate for alternative causes of LBP depending on the clinical presentation [27, 58].
Treatment of Vertebrogenic Low Back Pain
The current evidence supporting intraosseous BVN RFA comes from two large RCTs comparing BVN RFA to sham and “standard care” and from four single group cohort studies [57, 66–76], all demonstrating similar benefits. Each study used transpedicular access and bipolar RFA to target the BVN terminus at motion segments from L3 to S1 with MC1 and/or MC2. Single-arm meta-analysis of outcomes after intraosseous BVN RFA demonstrates that 64% (95% confidence interval [CI] 43–82%) and 75% (95% CI 63–85%) of participants report ≥50% pain reduction and ≥15-point Oswestry Disability Index (ODI) improvement at 12 months [52]. These improvements appear durable in the two studies that reported outcomes at 2 years and 5 years [57, 71, 74]. Although changes in chronic opioid use were less robust, interventional/surgical healthcare utilization decreased substantially after BVN RFA. For example, 49% of patients in a cohort study by Macadaeg et al. had received epidural steroid injections (ESI) prior to BVN RFA, but only 2% of these same patients received an ESI in the 12 months following treatment [76], with similar single digit utilization rates observed following BVN RFA for fusion surgery in the two RCTs at 2 and 5 years [57, 74].
Other than intraosseous BVN RFA, vertebrogenic pain has been treated with extraosseous epiduroscopic BVN/SVN laser ablation or bipolar RFA [77–79], intraosseous plasma rich growth factor [80], intraosseous injection of bioresorbable cement [81], and full endoscopic disc debridement surgery [82]. Oral therapies for presumed low grade infection affecting the discovertebral complex remain controversial [83–87]; however, research interest remains as a large RCTs are planned to further determine subpopulations who might benefit from antibiotic treatment [88]. Multiple studies have shown an association with paraspinal muscle quality, MC, and presence of low back pain [89–93], but it remains unknown how treatments to address paraspinal muscle deficits might impact those with vertebrogenic low back pain. A retrospective study of bracing for those with CLBP and MC1 reported short term pain relief [94]; however, RCTs have questioned the effectiveness of this intervention in general LBP populations [95].
Summary
Accumulated damage to the discovertebral complex may result in chemical and mechanical sensitization of endplate nocioceptors resulting in chronic vertebrogenic LBP.
Midline LBP, pain exacerbation by physical activity, sitting, and forward flexion are factors associated with treatment success after BVN RFA.
In appropriately selected patients, BVN RFA results in substantial reduction in pain and disability in the majority of those treated at 12 months, with similar long term outcomes at 5 years.
The presence of MC1 or MC2 is currently the best radiographic indicator of vertebrogenic pain. Outcomes after BVN RFA are not impacted by the volume of MC, location of MC, degree of disc degeneration, or presence/size of endplate defects. Patients with MC1 vs MC2 experience similar rates of success after BVN RFA.
Clinicians are encouraged to select patients for BVN RFA based upon the clinical and radiographic criteria used in published studies to date.
Future Research
Exploration of clinical, imaging, or other characteristics associated with vertebrogenic LBP may enable further progress in patient selection for BVN RFA. Enhanced diagnostics to isolate the source(s) of pain and further differentiate annular pain from vertebrogenic pain, such as MR spectroscopy and novel MRI sequences such as IDEAL and UTE may also be of value [96–100]. Evidence suggests a correlation between MC and increased endplate metabolic activity as detected by Single Positron Emission Computed Tomography (SPECT/CT) or bone scintigraphy [101, 102], but further study is necessary to know whether or not such findings are suggestive of vertebrogenic pain. Early research in serum biomarkers linked to vertebrogenic pain appears promising [103–105]. Finally, objective monitoring of real-life physical performance using wearables recently demonstrated the ability to identify kinematic and behavioral markers of spine disease [106–108]. Ongoing investigation in these areas may lead to more accurate phenotypes of Vertebrogenic LBP and influence treatment paradigms.
Contributor Information
Aaron Conger, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT, USA.
Matthew Smuck, Department of Orthopaedics, Stanford University, Redwood City, CA, USA.
Eeric Truumees, The University of Texas Dell Medical School, Ascension Texas Spine and Scoliosis, Austin, TX, USA.
Jeffrey C Lotz, Department of Orthopaedics, University of California San Francisco, San Francisco, CA, USA.
Michael J DePalma, Virginia iSpine Physicians, PC, Richmond, VA, USA.
Zachary L McCormick, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT, USA.
Funding sources: None.
Disclosure and conflicts of interest: Dr. McCormick has received research funding from Relievant Medsystems Inc., paid directly to the University of Utah; Dr. Conger has received research funding from Relievant Medsystems Inc., paid directly to the University of Utah; Dr. Smuck has received research funding from Relievant Medsystems Inc., paid directly to Stanford University; Dr. Lotz has received research funding from Relievant Medsystems, Inc., paid directly to the University of California at San Francisco, has stock ownership in Relievant Medsystems and is a consultant to Relievant Medsystems Inc.; Dr. Truumees has received research funding from Relievant Medsystems Inc., paid directly to The University of Texas Dell Medical School; Dr. DePalma has received research funding from Relievant Medsystems Inc.
Supplement sponsorship: This article appears as part of the supplement entitled “Vertebrogenic Pain and Basivertebral Nerve Radiofrequency Ablation” sponsored by Relievant Medsystems Inc.
References
- 1. Dillane JB, Fry J, Kalton G.. Acute back syndrome: A study from general practice. Br Med J 1966;2(5505):82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DePalma MJ, Ketchum JM, Saullo T.. What is the source of chronic low back pain and does age play a role? Pain Med 2011;12(2):224–33. [DOI] [PubMed] [Google Scholar]
- 3. Adams MA, Dolan P.. Intervertebral disc degeneration: Evidence for two distinct phenotypes. J Anat 2012;221(6):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Schepper EIT, Damen J, van Meurs JBJ, et al. The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 2010;35(5):531–6. [DOI] [PubMed] [Google Scholar]
- 5. Cheung KMC, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34(9):934–40. [DOI] [PubMed] [Google Scholar]
- 6. Videman T, Battié MC, Gibbons LE, Maravilla K, Manninen H, Kaprio J.. Associations between back pain history and lumbar MRI findings. Spine (Phila Pa 1976) 2003;28(6):582–8. [DOI] [PubMed] [Google Scholar]
- 7. Ohtori S, Miyagi M, Inoue G.. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: A review. Spine Surg Relat Res 2018;2(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MIV.. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997;350(9072):178–81. [DOI] [PubMed] [Google Scholar]
- 9. Fields AJ, Liebenberg EC, Lotz JC.. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J 2014;14(3):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antonacci MD, Mody DR, Heggeness MH.. Innervation of the human vertebral body. J Spinal Disord 1998;11(6):526–31. [PubMed] [Google Scholar]
- 11. Bailey JF, Liebenberg E, Degmetich S, Lotz JC.. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat 2011;218(3):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fields AJ, Ballatori A, Liebenberg EC, Lotz JC.. Contribution of the endplates to disc degeneration. Curr Mol Biol Rep 2018;4(4):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munir S, Freidin MB, Rade M, Määttä J, Livshits G, Williams FMK.. Endplate defect is heritable, associated with low back pain and triggers intervertebral disc degeneration: A longitudinal study from TwinsUK. Spine (Phila Pa 1976) 2018;43(21):1496–501. [DOI] [PubMed] [Google Scholar]
- 14. Johnson WEB, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S.. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum 2002;46(10):2658–64. [DOI] [PubMed] [Google Scholar]
- 15. Purmessur D, Cornejo MC, Cho SK, et al. Intact glycosaminoglycans from intervertebral disc-derived notochordal cell-conditioned media inhibit neurite growth while maintaining neuronal cell viability. Spine J 2015;15(5):1060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lama P, Le Maitre CL, Harding IJ, Dolan P, Adams MA.. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J Anat 2018;233(1):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC.. Pathobiology of modic changes. Eur Spine J 2016;25(11):3723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Degmetich S, Bailey JF, Liebenberg E, Lotz JC.. Neural innervation patterns in the sacral vertebral body. Eur Spine J 2016;25(6):1932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lotz JC, Fields AJ, Liebenberg EC.. The role of the vertebral end plate in low back pain. Glob Spine J 2013;3(3):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown MF, Hukkanen MVJ, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Jt Surg 1997;79-B(1):147–53. [DOI] [PubMed] [Google Scholar]
- 21. Fras C, Kravetz P, Mody DR, Heggeness MH.. Substance P-containing nerves within the human vertebral body. An immunohistochemical study of the basivertebral nerve. Spine J 2003;3(1):63–7. [DOI] [PubMed] [Google Scholar]
- 22. Fagan AB, Moore R, Roberts BV, Blumbergs P, Fraser R.. ISSLS prize winner: The innervation of the intervertebral disc: A quantitative analysis. Spine (Phila Pa 1976) 2003;28(23):2570–6. [DOI] [PubMed] [Google Scholar]
- 23. Michalik A, Conger A, Smuck M, Maus TP, McCormick ZL.. Intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebral body endplate low back pain: Current evidence and future directions. Pain Med 2021;22(suppl 1):S24–30. [DOI] [PubMed] [Google Scholar]
- 24. McCormick ZL, Sperry BA, , Boody BS, et al, . Pain location and exacerbating activities associated with treatment success following basivertebral nerve ablation: An aggregated cohort study of multicenter prospective clinical trial data. Pain Med 2022; (doi: 10.1093/pm/pnac069). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DePalma MJ, Ketchum JM, Saullo TR.. Multivariable analyses of the relationships between age, gender, and body mass index and the source of chronic low back pain. Pain Med 2012;13(4):498–506. [DOI] [PubMed] [Google Scholar]
- 26. Depalma MJ, Ketchum JM, Trussell BS, Saullo TR, Slipman CW.. Does the location of low back pain predict its source? PM R 2011;3(1):33–9. [DOI] [PubMed] [Google Scholar]
- 27. Cohen SP, Bhaskar A, Bhatia A, et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med 2020;45(6):424–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J 2007;16(10):1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fortin JD, Falco FJ.. The Fortin finger test: An indicator of sacroiliac pain. Am J Orthop (Belle Mead NJ) 1997;26(7):477–80. [PubMed] [Google Scholar]
- 30. Bailly F, Maigne J-Y, Genevay S, et al. Inflammatory pain pattern and pain with lumbar extension associated with Modic 1 changes on MRI: A prospective case-control study of 120 patients. Eur Spine J 2014;23(3):493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yong-Hing K, Kirkaldy-Willis WH.. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am 1983;14(3):491–504. [PubMed] [Google Scholar]
- 32. Lv B, Yuan J, Ding H, et al. Relationship between endplate defects, modic change, disc degeneration, and facet joint degeneration in patients with low back pain. Biomed Res Int 2019;2019:9369853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jensen TS, Kjaer P, Korsholm L, et al. Predictors of new vertebral endplate signal (Modic) changes in the general population. Eur Spine J 2010;19(1):129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Latif R, Imran S, Ahmad I, Ilyas MS, Aziz A, Zehra U.. Vertebral Endplate changes correlate with presence of cartilaginous endplate in the herniated disc tissue: factor predicting failure of conservative treatment. Asian Spine J 2021;16(2):212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Minetama M, Kawakami M, Teraguchi M, et al. Endplate defects, not the severity of spinal stenosis, contribute to low back pain in patients with lumbar spinal stenosis. Spine J 2021;22(3):370–8. [DOI] [PubMed] [Google Scholar]
- 36. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N.. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine (Phila Pa 1976) 1994;19(7):801–6. [DOI] [PubMed] [Google Scholar]
- 37. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR.. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166(1 Pt 1):193–9. [DOI] [PubMed] [Google Scholar]
- 38. Dudli S, Sing DC, Hu SS, Berven SH, et al. ISSLS PRIZE IN BASIC SCIENCE 2017: Intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J 2017;26(5):1362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teraguchi M, Hashizume H, Oka H, et al. Detailed subphenotyping of lumbar modic changes and their association with low back pain in a large population-based study: The Wakayama Spine Study. Pain Ther 2022;11(1):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weishaupt D, Zanetti M, Hodler J, et al. Painful lumbar disk derangement: Relevance of endplate abnormalities at MR imaging. Radiology 2001;218(2):420–7. [DOI] [PubMed] [Google Scholar]
- 41. Kuisma M, Karppinen J, Niinimäki J, et al. Modic changes in endplates of lumbar vertebral bodies: Prevalence and association with low back and sciatic pain among middle-aged male workers. Spine (Phila Pa 1976) 2007;32(10):1116–22. [DOI] [PubMed] [Google Scholar]
- 42. Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: A systematic review and meta-analysis. Am J Neuroradiol 2015;36(12):2394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lawan A, Crites Videman J, Battié MC.. The association between vertebral endplate structural defects and back pain: A systematic review and meta-analysis. Eur Spine J 2021;30(9):2531–48. [DOI] [PubMed] [Google Scholar]
- 44. McCormick ZL, Conger A, Hirsch J, et al. Magnetic resonance imaging characteristics associated with a success treatment outcome after basivertebral nerve ablation: An aggregated cohort study of multicenter prospective clinical trial data. Pain Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Conger A, Burnham T, Salazar F, et al. The effectiveness of radiofrequency ablation of medial branch nerves for chronic lumbar facet joint syndrome in patients selected by guideline-concordant dual comparative medial branch blocks. Pain Med 2020;21(5):902–9. [DOI] [PubMed] [Google Scholar]
- 46. Cohen SP, Hurley RW, Christo PJ, Winkley J, Mohiuddin MM, Stojanovic MP.. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain 2007;23(1):45–52. [DOI] [PubMed] [Google Scholar]
- 47. Stojanovic MP, Sethee J, Mohiuddin M, et al. MRI analysis of the lumbar spine: Can it predict response to diagnostic and therapeutic facet procedures? Clin J Pain 2010;26(2):110–5. [DOI] [PubMed] [Google Scholar]
- 48. Streitberger K, Müller T, Eichenberger U, Trelle S, Curatolo M.. Factors determining the success of radiofrequency denervation in lumbar facet joint pain: A prospective study. Eur Spine J 2011;20(12):2160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cohen SP, Stojanovic MP, Crooks M, et al. Lumbar zygapophysial (facet) joint radiofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: A multicenter analysis. Spine J 2008;8(3):498–504. [DOI] [PubMed] [Google Scholar]
- 50. Aggarwal A, Garg K.. Lumbar facet fluid—Does it correlate with dynamic instability in degenerative spondylolisthesis? A systematic review and meta-analysis. World Neurosurg 2021;149:53–63. [DOI] [PubMed] [Google Scholar]
- 51. Shinto K, Minamide A, Hashizume H, et al. Prevalence of facet effusion and its relationship with lumbar spondylolisthesis and low back pain: The Wakayama Spine Study. J Pain Res 2019;12:3521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Conger A, Burnham T, Clark T, Teramoto M, McCormick ZL.. The effectiveness of intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebrogenic low back pain: An updated systematic review with single arm meta-analysis. Pain Med 2022; (doi: 10.1093/pm/pnac070). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacVicar J, MacVicar AM, Bogduk N.. The prevalence of “pure” lumbar zygapophysial joint pain in patients with chronic low back pain. Pain Med 2021;22(1):41–8. [DOI] [PubMed] [Google Scholar]
- 54. Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N.. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med 1996;335(23):1721–6. [DOI] [PubMed] [Google Scholar]
- 55. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N.. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain 1994;58(2):195–200. [DOI] [PubMed] [Google Scholar]
- 56. Hurley RW, Adams MCB, Barad M, et al. Consensus practice guidelines on interventions for cervical spine (facet) joint pain from a multispecialty international working group. Reg Anesth Pain Med 2022;47(1):3–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fischgrund JS, Rhyne A, Macadaeg K, et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 2020;29(8):1925–34. [DOI] [PubMed] [Google Scholar]
- 58. McCormick ZL, DeFrancesch F, Loomba V, Moradian M, Bathina R, Rappard G.. Diagnostic value, prognostic value, and safety of provocation discography. Pain Med 2018;19(1):3–8. [DOI] [PubMed] [Google Scholar]
- 59. Wolfer LR, Derby R, Lee J-E, Lee S-H.. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician 2008;4;11(4):513–38. [PubMed] [Google Scholar]
- 60. Heggeness MH, Doherty BJ.. Discography causes end plate deflection. Spine (Phila Pa 1976) 1993;18(8):1050–3. [DOI] [PubMed] [Google Scholar]
- 61. Bartynski WS, Agarwal V, Khan AS, Bandos AI.. Motion characteristics of the functional spinal unit during lumbar disc injection (discography) including comparison between normal and degenerative levels. Pain Med 2021;22(8):1735–42. [DOI] [PubMed] [Google Scholar]
- 62. Peng B, Chen J, Kuang Z, Li D, Pang X, Zhang X.. Diagnosis and surgical treatment of back pain originating from endplate. Eur Spine J 2009;18(7):1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yoganandan N, Larson SJ, Pintar FA, Gallagher M, Reinartz J, Droese K.. Intravertebral pressure changes caused by spinal microtrauma. Neurosurgery 1994;35(3):415–21; discussion 421. [DOI] [PubMed] [Google Scholar]
- 64. Putzier M, Streitparth F, Hartwig T, Perka CF, Hoff EK, Strube P.. Can discoblock replace discography for identifying painful degenerated discs? Eur J Radiol 2013;82(9):1463–70. [DOI] [PubMed] [Google Scholar]
- 65. Alamin TF, Kim MJ, Agarwal V.. Provocative lumbar discography versus functional anesthetic discography: A comparison of the results of two different diagnostic techniques in 52 patients with chronic low back pain. Spine J 2011;11(8):756–65. [DOI] [PubMed] [Google Scholar]
- 66. De Vivo AE, D'Agostino G, D'Anna G, et al. Intra-osseous basivertebral nerve radiofrequency ablation (BVA) for the treatment of vertebrogenic chronic low back pain. Neuroradiology 2021;63(5):809–15. [DOI] [PubMed] [Google Scholar]
- 67. Truumees E, Macadaeg K, Pena E, et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Eur Spine J 2019;28(7):1594–602. [DOI] [PubMed] [Google Scholar]
- 68. Khalil JG, Smuck M, Koreckij T, et al. A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J 2019;19(10):1620–32. [DOI] [PubMed] [Google Scholar]
- 69. Fishchenko IV, Garmish AR, Kravchuk LD, Saponenko AI, Clinic CM.. Radiofrequency ablation of the basivertebral nerve in the treatment of chronic low back pain : Analysis of a small clinical series. Hir Pozvonochnika 2021;18(3):61–7. [Google Scholar]
- 70. Markman JD, Rhyne AL, Sasso RC, et al. Association between opioid use and patient-reported outcomes in a randomized trial evaluating basivertebral nerve ablation for the relief of chronic low back pain. Neurosurgery 2019;86(3):343–7. [DOI] [PubMed] [Google Scholar]
- 71. FISCHGRUND JS, RHYNE A, FRANKE J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 2-year results from a prospective randomized double-blind sham-controlled multicenter study. Int J Spine Surg 2019;13(2):110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Becker S, Hadjipavlou A, Heggeness MH.. Ablation of the basivertebral nerve for treatment of back pain: A clinical study. Spine J 2017;17(2):218–23. [DOI] [PubMed] [Google Scholar]
- 73. Fischgrund JS, Rhyne A, Franke J, Sasso R, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: A prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 2018;27(5):1146–56. [DOI] [PubMed] [Google Scholar]
- 74. Koreckij T, Kreiner S, Khalil JG, Smuck M, Markman J, Garfin S.. North American Spine Society Journal (NASSJ) Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain : 24-Month treatment arm results. North Am Spine Soc J 2021;8(October):100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Smuck M, Khalil J, Barrette K, et al. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-Month results. Reg Anesth Pain Med 2021;46(8):683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Macadaeg K, Truumees E, Boody B, et al. A prospective, single arm study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-Month results. N Am Spine Soc J 2020;3(September):100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim HS, Adsul N, Yudoyono F, et al. Transforaminal epiduroscopic basivertebral nerve laser ablation for chronic low back pain associated with modic changes: A preliminary open-label study. Pain Res Manag 2018;2018(Ddd):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim JY, Kim HS, Wu PH, Jang I-T.. Alleviating paravertebral muscle spasm after radiofrequency ablation treatment of hypersensitive basivertebral and sinuvertebral nerves for chronic discogenic back pain. Pain Physician 2021;24(6):E883–92. [PubMed] [Google Scholar]
- 79. Kim HS, Wu PH, Jang IT.. Lumbar degenerative disease part 1: Anatomy and pathophysiology of intervertebral discogenic pain and radiofrequency ablation of basivertebral and sinuvertebral nerve treatment for chronic discogenic back pain: A prospective case series and review of lite. Int J Mol Sci 2020;21(4):1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kirchner F, Pinar A, Milani I, Prado R, Padilla S, Anitua E.. Vertebral intraosseous plasma rich in growth factor (PRGF-Endoret) infiltrations as a novel strategy for the treatment of degenerative lesions of endplate in lumbar pathology: Description of technique and case presentation. J Orthop Surg Res 2020;15(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Masala S, Anselmetti GC, Marcia S, et al. Treatment of painful Modic type I changes by vertebral augmentation with bioactive resorbable bone cement. Neuroradiology 2014;56(8):637–45. [DOI] [PubMed] [Google Scholar]
- 82. Sairyo K, Maeda T, Yamashita K, et al. A new surgical strategy for the intractable chronic low back pain due to type 1 Modic change using transforaminal full-endoscopic disc cleaning (FEDC) surgery under the local anesthesia: A case report and literature review. J Med Invest 2021;68(1.2):1–5. [DOI] [PubMed] [Google Scholar]
- 83. Fritzell P, Welinder-Olsson C, Jönsson B, et al. Bacteria: Back pain, leg pain and Modic sign-a surgical multicentre comparative study. Eur Spine J 2019;28(12):2981–9. [DOI] [PubMed] [Google Scholar]
- 84. Kristoffersen PM, Bråten LCH, Vetti N, et al. Oedema on STIR modified the effect of amoxicillin as treatment for chronic low back pain with Modic changes-subgroup analysis of a randomized trial. Eur Radiol 2021;31(6):4285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bråten LCH, Grøvle L, Espeland A, AIM-Study Group, et al. Clinical effect modifiers of antibiotic treatment in patients with chronic low back pain and Modic changes—Secondary analyses of a randomised, placebo-controlled trial (the AIM study). BMC Musculoskelet Disord 2020;21(1):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bråten LCH, Rolfsen MP, Espeland A, et al. ; AIM Study Group. Efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM study): Double blind, randomised, placebo controlled, multicentre trial. BMJ 2019;367:l5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Albert HB, Sorensen JS, Christensen BS, Manniche C.. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): A double-blind randomized clinical controlled trial of efficacy. Eur Spine J 2013;22(4):697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Urquhart DM, Rosenfeld JV, van Tulder M, et al. Is antibiotic treatment effective in the management of chronic low back pain with disc herniation? Study protocol for a randomised controlled trial. Trials 2021;22(1):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Özcan-Ekşi EE, Turgut VU, Küçüksüleymanoğlu D, Ekşi MŞ.. Obesity could be associated with poor paraspinal muscle quality at upper lumbar levels and degenerated spine at lower lumbar levels: Is this a domino effect? J Clin Neurosci 2021;94:120–7. [DOI] [PubMed] [Google Scholar]
- 90. Özcan-Ekşi EE, Ekşi MŞ, Akçal MA.. Severe lumbar intervertebral disc degeneration is associated with modic changes and fatty infiltration in the paraspinal muscles at all lumbar levels, except for L1-L2: a cross-sectional analysis of 50 symptomatic women and 50 age-matched symptomatic men. World Neurosurg 2019;122:e1069–77. [DOI] [PubMed] [Google Scholar]
- 91. Atci IB, Yilmaz H, Samanci MY, Atci AG, Karagoz Y.. The prevalence of lumbar paraspinal muscle fatty degeneration in patients with Modic type I and I/II end plate changes. Asian Spine J 2020;14(2):185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ekşi MŞ, Özcan-Ekşi EE, Özmen BB, et al. Lumbar intervertebral disc degeneration, end-plates and paraspinal muscle changes in children and adolescents with low-back pain. J Pediatr Orthop B 2022;31(1):93–102. [DOI] [PubMed] [Google Scholar]
- 93. Ekşi MŞ, Özcan-Ekşi EE, Orhun Ö, Turgut VU, Pamir MN.. Proposal for a new scoring system for spinal degeneration: Mo-Fi-Disc. Clin Neurol Neurosurg 2020;198:106120. [DOI] [PubMed] [Google Scholar]
- 94. Boutevillain L, Bonnin A, Chabaud A, et al. Short-term pain evolution in chronic low back pain with Modic type 1 changes treated by a lumbar rigid brace: A retrospective study. Ann Phys Rehabil Med 2019;62(1):3–7. [DOI] [PubMed] [Google Scholar]
- 95. Annaswamy TM, Cunniff KJ, Kroll M, et al. Lumbar bracing for chronic low back pain: A randomized controlled trial. Am J Phys Med Rehabil 2021;100(8):742–9. [DOI] [PubMed] [Google Scholar]
- 96. Law T, Anthony M-P, Chan Q, et al. Ultrashort time-to-echo MRI of the cartilaginous endplate: Technique and association with intervertebral disc degeneration. J Med Imaging Radiat Oncol 2013;57(4):427–34. [DOI] [PubMed] [Google Scholar]
- 97. Chen KC, Tran B, Biswas R, et al. Evaluation of the disco-vertebral junction using ultrashort time-to-echo magnetic resonance imaging: Inter-reader agreement and association with vertebral endplate lesions. Skeletal Radiol 2016;45(9):1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vadapalli R, Mulukutla R, Vadapalli AS, Vedula RR.. Quantitative predictive imaging biomarkers of lumbar intervertebral disc degeneration. Asian Spine J 2019;13(4):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gornet MG, Peacock J, Claude J, et al. Magnetic resonance spectroscopy (MRS) can identify painful lumbar discs and may facilitate improved clinical outcomes of lumbar surgeries for discogenic pain. Eur Spine J 2019;28(4):674–87. [DOI] [PubMed] [Google Scholar]
- 100. Fields AJ, Ballatori A, Han M, et al. Measurement of vertebral endplate bone marrow lesion (Modic change) composition with water–fat MRI and relationship to patient-reported outcome measures. Eur Spine J 2021;30(9):2549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Russo VM, Dhawan RT, Dharmarajah N, Baudracco I, Lazzarino AI, Casey AT.. Hybrid bone single photon emission computed tomography imaging in evaluation of chronic low back pain: correlation with modic changes and degenerative disc disease. World Neurosurg 2017;104:816–23. [DOI] [PubMed] [Google Scholar]
- 102. Järvinen J, Niinimäki J, Karppinen J, Takalo R, Haapea M, Tervonen O.. Does bone scintigraphy show Modic changes associated with increased bone turnover? Eur J Radiol Open 2020;7:100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dudli S, Ballatori A, Bay-Jensen A-C, et al. Serum biomarkers for connective tissue and basement membrane remodeling are associated with vertebral endplate bone marrow lesions as seen on MRI (Modic changes). Int J Mol Sci 2020;21(11):3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li Y, Karppinen J, Cheah KSE, Chan D, Sham PC, Samartzis D.. Integrative analysis of metabolomic, genomic, and imaging-based phenotypes identify very-low-density lipoprotein as a potential risk factor for lumbar Modic changes. Eur Spine J 2021;31(3):735–45. [DOI] [PubMed] [Google Scholar]
- 105. Karppinen J, Koivisto K, Ketola J, et al. Serum biomarkers for Modic changes in patients with chronic low back pain. Eur Spine J 2021;30(4):1018–27. [DOI] [PubMed] [Google Scholar]
- 106. Tomkins-Lane C, Norden J, Sinha A, Hu R, Smuck M.. Digital biomarkers of spine and musculoskeletal disease from accelerometers: Defining phenotypes of free-living physical activity in knee osteoarthritis and lumbar spinal stenosis. Spine J 2019;19(1):15–23. [DOI] [PubMed] [Google Scholar]
- 107. Odonkor C, Kuwabara A, Tomkins-Lane C, et al. Gait features for discriminating between mobility-limiting musculoskeletal disorders: Lumbar spinal stenosis and knee osteoarthritis. Gait Posture 2020;80:96–100. [DOI] [PubMed] [Google Scholar]
- 108. Tomkins-Lane C, Sun R, Muaremi A, et al. Objective features of sedentary time and light activity differentiate people with low back pain from healthy controls: A pilot study. Spine J 2022;22(4):629–34. [DOI] [PubMed] [Google Scholar]