Abstract
Objective
Multiple studies have demonstrated the safety and effectiveness of basivertebral nerve radiofrequency ablation (BVN RFA) for improving low back pain related to the vertebral endplate. However, the influence of patient demographic and clinical characteristics on treatment outcome is unknown.
Design
Pooled cohort study of three clinical trials of patients with vertebral endplate pain identified by Type 1 and/or Type 2 Modic changes and a correlating presentation of anterior spinal element pain.
Setting
Thirty-three global study centers.
Subjects
Patients (n = 296) successfully treated with BVN RFA.
Methods
Participant demographic and clinical characteristics were analyzed with stepwise logistic regression to identify predictors of treatment success. Three definitions of treatment success were defined: 1) ≥50% visual analog scale pain improvement, 2) ≥15-point Oswestry Disability Index (ODI) improvement, and 3) ≥50% visual analog scale or ≥15-point ODI improvement from baseline.
Results
Low back pain of ≥5 years’ duration and higher ODI scores at baseline increased the odds of treatment success, whereas baseline opioid use and higher Beck Depression Inventory scores reduced these odds. However, the three regression models demonstrated receiver-operating characteristics of 62–70% areas under the curve, and thus, limited predictive capacity.
Conclusions
This analysis identified no demographic or clinical characteristic that meaningfully increased or reduced the odds of treatment success from BVN RFA. On the basis of these findings and the high response rates from the three analyzed trials, we recommend the use of objective imaging biomarkers (Type 1 and/or 2 Modic changes) and a correlating presentation of anterior spinal element pain to determine optimal candidacy for BVN RFA.
Keywords: Vertebrogenic Pain, Endplate, Low Back
Introduction
Low back pain (LBP) is a highly prevalent condition that is associated with substantial direct health care costs and decreased productivity in the workplace [1,2]. Many treatments for LBP are associated with small effect sizes [3], likely because of the inclusion of heterogenous patient populations for whom a specific diagnosis has not been determined. More recently, vertebral endplate pain (VEP) has been identified as a source of LBP. The U.S. Centers for Disease Control and Prevention recognized the need for greater granularity in differentiating types of LBP when they added the International Classification of Diseases (ICD)-10CM code M54.51 in October 2021. Pathological changes and sensitization of the basivertebral nerve (BVN) result from degeneration of the disc/vertebral endplate complex with exchange of inflammatory material through defects in this barrier [4–9]. Magnetic resonance imaging findings of Type 1 and/or Type 2 Modic changes correlate with the histopathological changes observed in patients with VEP [5,6,10–15].
Given that the BVN provides the majority of nociceptive input to the vertebral endplates [7,8,16–18], targeted ablation of this structure should result in a reduction of VEP symptoms. Two randomized controlled trials (RCTs) and a single-arm prospective cohort study were conducted to determine the safety, efficacy and effectiveness of BVN radiofrequency ablation (BVN RFA) for the treatment of lumbosacral VEP identified through the presence of Type 1 and/or Type 2 Modic changes on magnetic resonance imaging and a correlating presentation of anterior spinal element pain [19–25]. The three studies had the same pre-specified primary endpoint of mean Oswestry Disability Index (ODI) reduction at 3 months after BVN RFA. Aggregate results for these three studies demonstrate a mean ODI improvement from baseline to 3 months after ablation of 23.6 points (standard deviation [SD] 16.5; 95% confidence interval [CI] 21.7–21.5) and visual analog scale (VAS) decreases of 3.33 cm (SD 2.55; 95% CI 3.04–3.62) at 3 months after ablation in successfully targeted BVN RFA patients [19,21,22]. Response rates were 67.0% (95% CI 61.3–72.4%) for a ≥15-point improvement in ODI and 54.5% (95% CI 48.6–60.3%) for a ≥50% VAS reduction at 3 months after BVN RFA for the aggregate cohort [19,21,22]. Outcomes were sustained at 12 and 24 months after ablation [19,22–25], and significant incremental changes from baseline were noted from 12 and 24 months to 5 years after ablation [26]. These studies’ aggregate results demonstrate the effectiveness of BVN RFA for significantly improving pain and functional outcomes in patients selected objectively by Modic changes and pain characteristics suggestive of VEP whose LBP was refractory to ongoing medical management [19–27].
Patient characteristics, including smoking, obesity, age, anxiety, depression, and stress, have been reported to adversely impact LBP for overall patient disability [28–31]. Although the relationships of various clinical characteristics with the outcomes of treatments for specific causes of LBP have been investigated [32–37], the influence of such factors on the treatment outcome of BVN RFA for individuals with VEP has not been defined. The present study cataloged and analyzed participant characteristics from three prospective clinical trials of BVN RFA for VEP to assess factors that might predict treatment success.
Methods
The present study was an analysis of pooled cohort data from three prospective clinical trials sponsored by Relievant Medsystems Inc. (Minneapolis, MN). Study patients were enrolled from October 2011 through February 2019 at a total of 33 academic and private practice pain and spine centers in the United States and Europe. Aggregate data from three trials were analyzed: 1) the original investigation, an RCT conducted for the U.S. Food and Drug Administration, which included 147 BVN RFA–treated patients and 78 sham controls [19]; 2) a second RCT, which included 66 patients who were randomized and treated with BVN RFA and 74 who were randomized to standard care control, of whom 61 crossed over to BVN RFA treatment [20,22]; and 3) a single-arm, prospective study that included 48 patients treated with BVN RFA [21,23]. Each study was approved by an Institutional Review Board (Western IRB # PRO20111346, Schulman IRB #201702680/ADVARRA IRB# PRO00026311, and Schulman IRB # 201706803/Advarra IRB #Pro000226859, respectively), with informed consent and privacy authorization by study patients. Each study was registered on ClinicalTrials.gov (trial registration numbers NCT01446419, NCT03246061, and NCT03266107, respectively). No clinical sites or study patients were contacted for this retrospective analysis. All data used in this analysis were deidentified and are unable to be traced to individual patients.
All patients enrolled in the study displayed either Type 1 and/or Type 2 Modic changes as an objective biomarker for VEP. Inclusion and exclusion criteria to rule out other primary LBP etiologies were similar among all three studies and can be found within previously published articles [19–22]. See Table 1 for a complete listing of inclusion and exclusion criteria for the three studies. In all three studies, patients with refractory, chronic LBP and Type 1 and/or Type 2 Modic changes were treated with BVN RFA at each vertebral body level (L3–S1) with Modic changes present. BVN RFA was conducted with image guidance with an ablation target at the midpoint of each vertebral body in an anterior-posterior view at a point approximately 50% from the posterior wall in a lateral view (40–60% [19] range used in the initial RCT and an adjusted range of 30–50% [21,22] of the diameter of the vertebral body in a lateral view used in the second RCT with enhanced target success) at the stem of the BVN for the L3–L5 levels and at approximately 50% of the diameter of the S1 vertebral segment where BVN capture is most likely on the basis of foundational anatomic work [18]. The Intracept® System (Relievant Medsystems, Minneapolis, MN USA) was used for all BVN RFA treatments. The full procedure has been described previously [19,21].
Table 1.
Inclusion and exclusion criteria for the three studies used in this aggregated analysis
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
MRI = magnetic resonance imaging; BMI = body mass index.
Prior regression analyses of treatment and control arm randomized patients with a minimum response threshold of ODI ≥10-point improvement and VAS ≥1.5-cm improvement found that treatment allocation (BVN RFA vs sham or standard nonsurgical care control) was predictive of response. With this understanding, only patients who received BVN RFA and for whom targeting success was achieved, with a minimum follow-up of 3 months, were included in the regression analysis for the present study. Targeting success was evaluated in all three studies by the same independent radiologist, who confirmed adequate overlap of the BVN by the BVN RFA lesion for each level treated [19–23].
Demographic and Clinical Characteristics Evaluated
Demographic and clinical characteristics within the pooled cohort were cataloged on the basis of data collected in the three clinical trials and a requirement that each candidate factor was available in at least 90% of the study patients who underwent BVN RFA. Such factors were also cataloged in control patients from the two RCTs for input into the regression analysis to assess the impact of the treatment allocation (described previously in the preceding “Methods” section). These factors included age, sex, marital status, employment status, duration of pain, history of opioid use, depression, anxiety, facet joint arthropathy, radicular pain/weakness, and baseline scores for body mass index, Beck Depression Index (BDI), ODI, VAS, Short-Form Survey 36 physical component and mental components, Modic type 1, Modic type 2, and number of treated levels (1–4).
One factor, income, was omitted from the model because more than 10% of patients refused to report their income level. Two variables were further condensed into binary variables to reduce the number of variables and increase the statistical power. Employment status was used to define a binary “employed” variable, where the responses “Working Full-Time,” “Working Part-Time,” and “Retired” were counted as employed, and the responses “Unemployed,” “Not Working Due to Back Pain,” and “Other” were counted as unemployed. Marital status was used to define a binary “married” variable, where the response “Married” was counted as married, and the responses “Divorced,” “Separated,” “Single,” and “Widowed” were counted as not married.
Definition of Treatment Success
To investigate the predictive value of demographic and clinical characteristics for treatment success at 3 months after BVN RFA, “treatment success” was evaluated on the basis of three definitions of response: 1) ≥50% VAS pain reduction from baseline, 2) ≥15-point improvement in ODI score from baseline, and 3) ≥50% VAS or ≥15-point ODI improvement from baseline. Such thresholds are commonly used in the LBP treatment literature and considered to be rigorous [38,39].
Statistical Analysis
Descriptive statistics were calculated for the selected demographic and clinical factors in the successfully treated BVN RFA population (n = 296). For continuous variables, the sample size (n), mean, standard deviation, median, minimum, and maximum values were calculated. For categorical variables, the number and percentage in each category were calculated. A Wilcoxon rank-sum test was used to explore the relationship between continuous variables and response, while a Fisher’s exact test was used for categorical variables (Table 1).
Through the use of the investigator-preselected candidate factors from available data in the three studies, a stepwise logistic regression was used to identify the best predictors of response in successfully treated BVN RFA patients for each of the three responder definitions (as discussed in the “Definition of Treatment Success” section). The stepwise regression combined forward-selection and backward-elimination regression techniques. The stepwise regression began by entering the intercept for the model. The stepwise regression models fit for the present analysis used an entry criterion of 0.05 and a stay criterion of 0.10. For each subsequent iteration, the predictor with the smallest P value, which was less than the prespecified 0.05 entry criterion, was entered into the model. After the predictors’ entry, the model was fit, and each predictor in the model was assessed for statistical significance. To stay in the model, each predictor was required to have a P value of less than the prespecified 0.10 stay criterion. These iterations continued until no further predictors were added into or removed from the model. All descriptive statistics and modeling were carried out in SAS version 9.4 (SAS, Cary, NC).
With the logistic regression model being fit, estimates of the dependent variables (predictors) were used to predict the probability of the binary outcome, in this case treatment success. In translating the probability of success into a binary yes/no, the threshold number of 0.5 was chosen. In this analysis, if the predicted probability of success from the model was greater than 0.5 for an individual patient in the study cohort, that patient was predicted as a treatment success. If the predicted probability of success was less than 0.5, that patient was predicted as a treatment failure (nonsuccess).
The predicted success/failure of each participant from the model estimates was compared with the known actual success/failure from the patients’ study data. A count of the number of patients who are true positives (successes), true negatives (failures), false positives, and false negatives (based on their model predicted values and actual values) was performed. The sensitivity of that given threshold is the rate of true positives, while the specificity is the rate of true negatives. The receiver-operating characteristics curve (ROC) graphs depict the sensitivity on the y-axis and (1 minus specificity) on the x-axis for various values of the predicted probability threshold. The regression model had good discrimination and was well calibrated (observed to expected ratios, 1.00) in the development cohort and in the validation cohort.
The final step in validating the model was to interpret the area under the ROC curve (AUC) or the rate of successful classification from the logistic regression model. This value can range from 0 to 1, where 0 indicates a perfectly inaccurate model classification of treatment success, and 1 indicates a perfectly accurate model classification of treatment success. In general, an AUC value of 0.5 indicates no discrimination between treatment success/failure by the fitted logistic regression model. AUC values between 0.5 and 0.7 indicate some predictive ability, values between 0.7 and 0.8 indicate acceptable predictive ability, values between 0.8 and 0.9 are considered to indicate excellent predictive ability, and values more than 0.9 are considered to indicate outstanding predictive ability [40].
Results
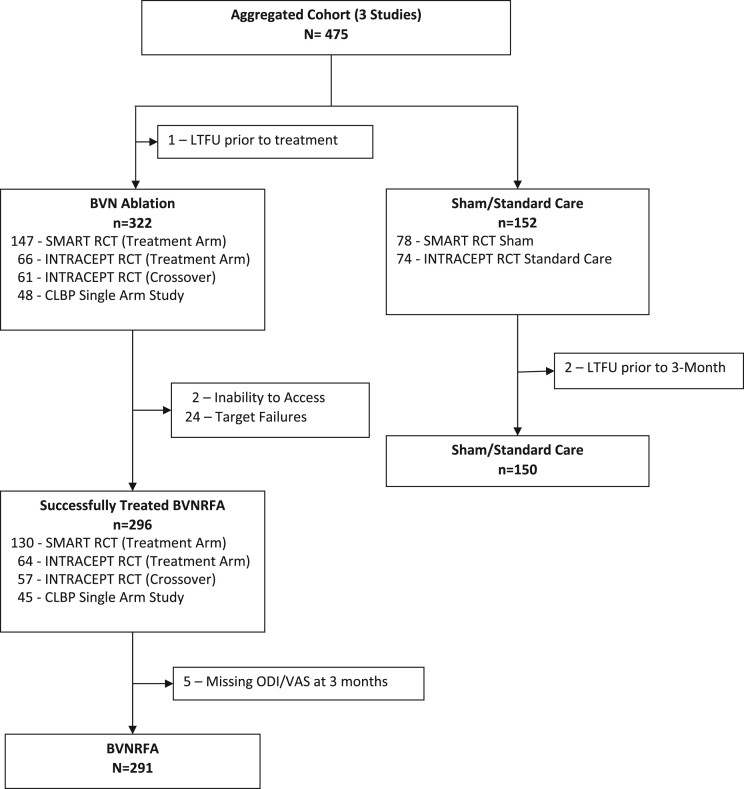
A total of 475 patients from the three clinical trials had a minimum variable dataset and were included in the analysis of potential predictors for the model (322 BVN RFA, including 61 control patients who crossed to active treatment, and 152 controls). Of the BVN RFA group, 296 were treated successfully and comprised the cohort for the regression analysis. Of these, 291 patients had a minimum of all predictors and both an ODI and VAS at 3 months for the combined response definition. See Figure 1, the CONSORT diagram.
Figure 1.
CONSORT diagram of the aggregate cohort included in the regression analysis. A total of 475 patients from the three clinical trials had a minimum predictor dataset and were included in an analysis of potential predictors for the model (322 BVN RFA, including 61 control patients who crossed to active treatment, and 152 controls). Of the BVN RFA group, 291 were treated successfully, had a minimum of a 3-month follow-up with ODI and VAS scores collected, and comprised the cohort for the regression analysis.
Table 2 reports the demographic data for the n = 296 regression cohort, as well as stratification by responder vs nonresponder status according to primary pain and functional improvement definitions of treatment success: 1) ≥50% VAS improvement, 2) ≥15-point ODI improvement, and 3) ≥50% VAS or ≥15-point ODI improvement. Among the regression cohort, the average age was 48 years (SD 10), with 53% male, 71% married, and 90% employed (as defined in the “Methods” section). Sixty-nine percent (69%) of patients reported LBP duration of ≥5 years. Approximately one fourth of patients had a history of anxiety or depression, at 20% and 22%, respectively, and 28% were taking opioids at baseline. Pain was severe and function was moderate to severe for disability impact at baseline, with a mean VAS score of 6.8 ± 1.3 and a mean ODI score of 44.5 ± 11.2. Patients reported an average BDI score of 6.7 ± 5.3 at baseline.
Table 2.
Descriptive statistics for the aggregate BVN RFA cohort from the three included studies (n= 296) and for patients with the minimum dataset for each response definition regression model
| Characteristic | All Successfully Treated Subjects | Responder (VAS reduction ≥50%) | Nonresponder (VAS reduction <50%) | P Value Wilcoxon (t Test) or Fisher’s Exact Test | Responder (ODI reduction ≥15 points) | Nonresponder (ODI reduction <15 points) | P Value Wilcoxon (t Test) or Fisher’s Exact Test |
|---|---|---|---|---|---|---|---|
| Age, years | 0.0292 | ||||||
| N | 296 | 159 | 133 | 195 | 96 | ||
| Mean (SD) | 47.9 (10.2) | 49.1 (9.9) | 46.5 (10.4) | 48.1 (9.9) | 47.5 (10.9) | 0.6465 | |
| Median | 47.8 | 49.0 | 46.1 | 48.2 | 46.9 | ||
| Min, max | 25.8, 71.0 | 25.8, 69.9 | 26.4, 71.0 | 25.8, 69.9 | 26.4, 71.0 | ||
| Gender | 1.0000 | 0.0174 | |||||
| Male | 53.4% (158) | 54.2% (84/155) | 45.8% (71/155) | . | 60.6% (94/155) | 39.4% (61/155) | . |
| Female | 46.6% (138) | 54.7% (75/137) | 45.3% (62/137) | . | 74.3% (101/136) | 25.7% (35/136) | . |
| Baseline BMI | |||||||
| N | 296 | 159 | 133 | 195 | 96 | ||
| Mean (SD) | 26.8 (6.5) | 27.3 (5.9) | 26.3 (7.3) | 0.4309 | 27.0 (6.4) | 26.5 (6.9) | 0.9539 |
| Median | 27.3 | 27.3 | 27.1 | 27.2 | 27.3 | ||
| Min, max | 0.0, 40.9 | 0.0, 40.9 | 0.0, 38.7 | 0.0, 40.9 | 0.0, 38.7 | ||
| Married | 0.4374 | 0.4111 | |||||
| No | 29.2% (86) | 50.6% (43/85) | 49.4% (42/85) | . | 63.5% (54/85) | 36.5% (31/85) | . |
| Yes | 70.8% (209) | 56.3% (116/206) | 43.7% (90/206) | . | 68.8% (141/205) | 31.2% (64/205) | . |
| Employed | 0.0520 | 0.6839 | |||||
| No | 10.5% (31) | 36.7% (11/30) | 63.3% (19/30) | . | 63.3% (19/30) | 36.7% (11/30) | . |
| Yes | 89.5% (265) | 56.5% (148/262) | 43.5% (114/262) | . | 67.4% (176/261) | 32.6% (85/261) | . |
| History of opioid use | 0.5150 | 0.0515 | |||||
| No | 71.6% (212) | 55.7% (117/210) | 44.3% (93/210) | . | 70.5% (148/210) | 29.5% (62/210) | . |
| Yes | 28.4% (84) | 51.2% (42/82) | 48.8% (40/82) | . | 58.0% (47/81) | 42.0% (34/81) | . |
| History of depression | 0.6741 | 1.0000 | |||||
| No | 78.0% (231) | 53.7% (122/227) | 46.3% (105/227) | . | 66.8% (151/226) | 33.2% (75/226) | . |
| Yes | 22.0% (65) | 56.9% (37/65) | 43.1% (28/65) | . | 67.7% (44/65) | 32.3% (21/65) | . |
| History of anxiety | 0.6610 | 0.4340 | |||||
| No | 80.1% (237) | 55.1% (129/234) | 44.9% (105/234) | . | 65.8% (154/234) | 34.2% (80/234) | . |
| Yes | 19.9% (59) | 51.7% (30/58) | 48.3% (28/58) | . | 71.9% (41/57) | 28.1% (16/57) | . |
| Duration of pain ≥5 years “yes” | 0.0016 | 0.4235 | |||||
| No | 30.7% (91) | 40.7% (37/91) | 59.3% (54/91) | . | 63.7% (58/91) | 36.3% (33/91) | . |
| Yes | 69.3% (205) | 60.7% (122/201) | 39.3% (79/201) | . | 68.5% (137/200) | 31.5% (63/200) | . |
| Facet arthropathy | 0.4032 | 0.4486 | |||||
| No | 59.1% (175) | 56.6% (98/173) | 43.4% (75/173) | . | 68.8% (119/173) | 31.2% (54/173) | . |
| Yes | 40.9% (121) | 51.3% (61/119) | 48.7% (58/119) | . | 64.4% (76/118) | 35.6% (42/118) | . |
| Radicular pain/weakness | 1.0000 | 0.6212 | |||||
| No | 93.2% (275) | 54.8% (149/272) | 45.2% (123/272) | . | 66.8% (181/271) | 33.2% (90/271) | . |
| Yes | 6.8% (20) | 52.6% (10/19) | 47.4% (9/19) | . | 73.7% (14/19) | 26.3% (5/19) | . |
| Baseline BDI | |||||||
| N | 296 | 159 | 133 | 195 | 96 | ||
| Mean (SD) | 6.7 (5.3) | 6.1 (5.0) | 7.4 (5.5) | 0.0287 | 6.1 (4.9) | 7.9 (5.8) | 0.0143 |
| Median | 5.0 | 5.0 | 6.0 | 5.0 | 6.5 | ||
| Min, max | 0.0, 23.0 | 0.0, 23.0 | 0.0, 23.0 | 0.0, 23.0 | 0.0, 23.0 | ||
| Baseline ODI | |||||||
| N | 296 | 159 | 133 | 195 | 96 | ||
| Mean (SD) | 44.5 (11.2) | 44.2 (10.9) | 44.6 (11.6) | 0.8885 | 46.2 (11.6) | 40.5 (9.4) | <0.0001 |
| Median | 42.0 | 42.0 | 42.0 | 44.0 | 38.0 | ||
| Min, max | 30.0, 88.0 | 30.0, 88.0 | 30.0, 76.0 | 30.0, 88.0 | 30.0, 70.0 | ||
| Baseline VAS | |||||||
| N | 296 | 159 | 133 | 195 | 96 | ||
| Mean (SD) | 6.8 (1.3) | 6.7 (1.2) | 6.9 (1.4) | 0.1996 | 6.8 (1.3) | 6.7 (1.3) | 0.6139 |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | ||
| Min, max | 4.0, 10.0 | 4.0, 10.0 | 4.0, 10.0 | 4.0, 10.0 | 4.0, 10.0 | ||
| Baseline SF-36 PCS Score | |||||||
| N | 295 | 159 | 133 | 195 | 96 | ||
| Mean (SD) | 32.6 (7.1) | 32.5 (6.8) | 32.9 (7.5) | 0.5426 | 31.9 (6.7) | 34.4 (7.8) | 0.0028 |
| Median | 32.3 | 31.9 | 33.4 | 31.3 | 35.0 | ||
| Min, max | 14.8, 48.1 | 17.2, 48.0 | 14.8, 48.1 | 14.8, 48.0 | 17.4, 48.1 | ||
| Baseline SF-36 MCS Score | |||||||
| N | 295 | 159 | 133 | 0.9114 | 195 | 96 | |
| Mean (SD) | 52.9 (10.0) | 53.1 (9.7) | 52.9 (10.3) | 53.5 (9.8) | 52.0 (10.3) | 0.2642 | |
| Median | 55.2 | 55.0 | 56.0 | 55.7 | 54.3 | ||
| Min, max | 19.8, 69.8 | 19.8, 69.8 | 22.2, 68.9 | 19.8, 69.8 | 26.9, 69.1 | ||
| Modic Type I | 0.6322 | 0.3104 | |||||
| No | 39.5% (117) | 52.6% (61/116) | 47.4% (55/116) | . | 63.5% (73/115) | 36.5% (42/115) | . |
| Yes | 60.5% (179) | 55.7% (98/176) | 44.3% (78/176) | . | 69.3% (122/176) | 30.7% (54/176) | . |
| Modic Type II | 0.7247 | 0.2122 | |||||
| No | 51.7% (153) | 55.6% (84/151) | 44.4% (67/151) | . | 70.7% (106/150) | 29.3% (44/150) | . |
| Yes | 48.3% (143) | 53.2% (75/141) | 46.8% (66/141) | . | 63.1% (89/141) | 36.9% (52/141) | . |
| Number of treated levels | |||||||
| N | 296 | 159 | 133 | 195 | 96 | ||
| Mean (SD) | 2.2 (0.5) | 2.3 (0.5) | 2.2 (0.5) | 0.4838 | 2.2 (0.5) | 2.3 (0.5) | 0.4553 |
| Median | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | ||
| Min, max | 2.0, 4.0 | 2.0, 4.0 | 2.0, 4.0 | 2.0, 4.0 | 2.0, 4.0 |
Min= minimum; max= maximum; BMI= body mass index; SF-36= Short-Form-36; PCS= Physical Component Score; MCS= Mental Component Score.
Descriptive statistics for the patients successfully treated with BVN RFA from the three included studies (n= 296), and for patients with the minimum data set for each response definition regression model, are shown.
For categorical variables, the provided P values come from a Fisher’s exact test. For the continuous variables, the provided P values come from a nonparametric Wilcoxon rank-sum test and a two-sample t test in parentheses. Analysis was conducted in SAS version 9.4.
Table 3 shows all variables that were not included in each of the three models after the stepwise selection process, along with the P value associated with the individual score statistics for each variable. The P values in Table 2 are compared with the entry criterion of 0.05 and stay criterion of 0.10 for determination of whether they should be entered into the model during the stepwise selection process.
Table 3.
Nonpredictive variables removed from the final regression model
| Variable | Definition 1 Response Threshold | Definition 2 Response Threshold | Definition 3 Response Threshold |
|---|---|---|---|
| ≥50% VAS improvement | ≥15 ODI improvement | ≥50% VAS or ≥15 ODI improvement | |
| P Value | P Value | P Value | |
| (n=292) | (n=291) | (n=292) | |
| Age | 0.1933 | 0.5762 | 0.5024 |
| Sex | 0.6798 | 0.0927 | 0.2686 |
| Married | 0.5416 | 0.7837 | 0.8384 |
| Pain duration ≥5 years | Included in the final model | 0.5994 | 0.2003 |
| History of depression | 0.2102 | 0.3024 | 0.166 |
| History of anxiety | 0.9015 | 0.2146 | 0.2214 |
| History of opioid use | 0.886 | Included in the final model | 0.239 |
| Employed | 0.1874 | 0.5707 | 0.6254 |
| Facet arthropathy | 0.3546 | 0.7962 | 0.8707 |
| Radicular pain/weakness | 0.7015 | 0.4162 | 0.4715 |
| Baseline BMI | 0.2058 | 0.5708 | 0.6929 |
| Baseline BDI | Included in the final model | Included in the final model | Included in the final model |
| Baseline VAS score | 0.3585 | 0.1853 | 0.2089 |
| Baseline ODI score | 0.8449 | Included in the final model | Included in the final model |
| Baseline SF-36 PCS score | 0.4977 | 0.3379 | 0.7675 |
| Baseline SF-36 MCS score | 0.2175 | 0.795 | 0.6659 |
| Modic Type 1 | 0.5802 | 0.6332 | 0.7288 |
| Modic Type 2 | 0.6146 | 0.49 | 0.5947 |
| Number of treated levels | 0.8768 | 0.5385 | 0.3919 |
SF-36= Short-Form-36; PCS= Physical Component Score; MCS= Mental Component Score.
Variables that were not selected for the final model based on the stepwise logistic regression approach with each definition of response are shown. Except as noted, these predictors were not considered statistically significant predictors when fitting the regression model with an entry P value of 0.05 and a stay P value of 0.10.
Table 4 provides an interpretation for the area under the curve (AUC) range of values for the ROC curve, with a value of 0.5 indicating no discrimination between treatment success and failure by the fitted logistic regression model. AUC values between 0.5 and 0.7 indicate some predictive ability, values between 0.7 and 0.8 indicate acceptable predictive ability, values between 0.8 and 0.9 are considered to indicate excellent predictive ability, and values more than 0.9 are considered to indicate outstanding predictive ability [40].
Table 4.
AUC value range interpretations
| AUC Value | Interpretation |
|---|---|
| 0.5 or below | No discrimination between treatment success/failure by the fitted logistic regression model |
| 0.5 to <0.7 | Some predictive ability |
| 0.7 to <0.8 | Acceptable predictive ability |
| 0.8 to <0.9 | Excellent predictive ability |
| More than 0.9 | Outstanding predictive ability |
Adapted from Mandrekar [40].
Table provides the interpretation for the AUC range of values for the ROC. A value of 0.5 indicates no discrimination between treatment success and failure by the fitted logistic regression model. AUC values between 0.5 and 0.7 indicate some predictive ability, values between 0.7 and 0.8 indicate acceptable predictive ability, values between 0.8 and 0.9 are considered to indicate excellent predictive ability, and values more than 0.9 are considered to indicate outstanding predictive ability [40].
Table 5 reports the result of the stepwise logistic regression analyses with the response definition ≥50% VAS improvement. The final logistic regression model included pain duration ≥5 years and baseline BDI score. Pain duration of ≥5 years increased the odds of treatment success. Conversely, a higher baseline BDI score (greater depression symptoms) decreased the odds of treatment success. The demonstrated ROC curve was 62%, for limited predictive ability. Figure 2 shows the ROC curve for the model.
Table 5.
Predictive model from the final selected model following stepwise logistic regression (Response Definition 1)
| Model | Variable Included | Odds Ratio | P Value | Pseudo R2 | Area Under ROC Curve |
|---|---|---|---|---|---|
|
Pain duration ≥5 years (yes vs no) | 2.211 | 0.0022 | ||
| 0.05 | 0.62 | ||||
| Baseline BDI | 0.954 | 0.0403 |
Final candidate predictors for the final model are shown: Pain duration and baseline BDI score demonstrated a P value <0.05 with Response Definition 1 (≥50% VAS improvement). Of the variables examined, pain duration ≥5 years increased the odds of treatment success, whereas higher baseline BDI score (greater depression symptoms) decreased the odds of treatment success. The AUC for this model is 0.62, for limited predictive ability.
Figure 2.
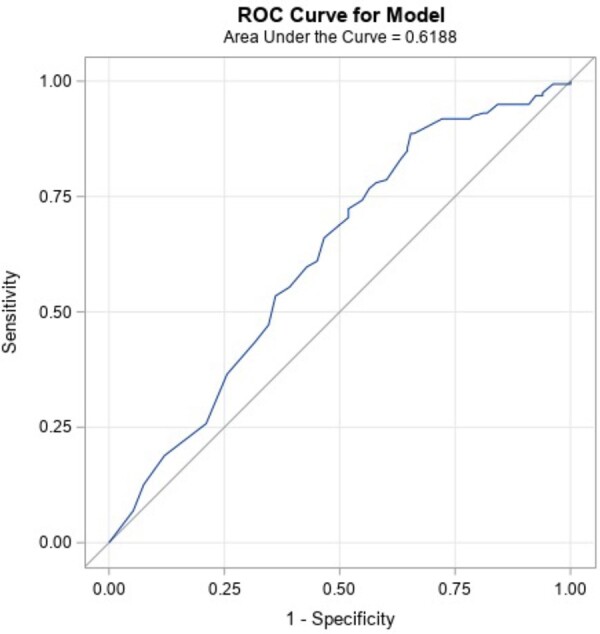
ROC curve of the predictive model (Response Definition 1). The ROC curve for the model fit with Response Definition 1 (≥50% VAS improvement). ROC curves plot the sensitivity against 1 minus specificity, such that a perfect diagnostic would have an AUC of 1.0 (100%). The AUC for this model is 0.62, for limited predictive/diagnostic ability.
Table 6 shows the result of the stepwise logistic regression analyses with the response definition ≥15-point ODI improvement. The final logistic regression model included history of opioid use, baseline BDI score, and baseline ODI score. Having a higher baseline ODI score (greater functional impairment related to LBP) increased the odds of treatment success, whereas a history of opioid use and higher baseline BDI scores (greater depression symptoms) decreased the odds of treatment success. The demonstrated ROC curve was 70%, for borderline acceptable predictive ability. Figure 3 shows the ROC curve for the model.
Table 6.
Predictive model from the final selected model following stepwise logistic regression (Response Definition 2)
| Model | Variable Included | Odds Ratio | P Value | Pseudo R2 | Area Under ROC Curve |
|---|---|---|---|---|---|
|
History of opioid use (yes vs no) | 0.544 | 0.0424 | ||
| Baseline BDI | 0.943 | 0.0203 | 0.10 | 0.70 | |
| Baseline ODI | 1.062 | <0.0001 |
Final candidate predictors for the final model are shown: Opioid use, baseline BDI score, and baseline ODI demonstrated a P value <0.05 with Response Definition 2 (≥15-point ODI improvement). Of the variables examined, higher baseline ODI score (greater functional impairment related to LBP) increased the odds of treatment success, whereas history of opioid use and higher baseline BDI score (greater depression symptoms) decreased the odds of treatment success. The AUC for this model is 0.70, for borderline acceptable predictive ability.
Figure 3.
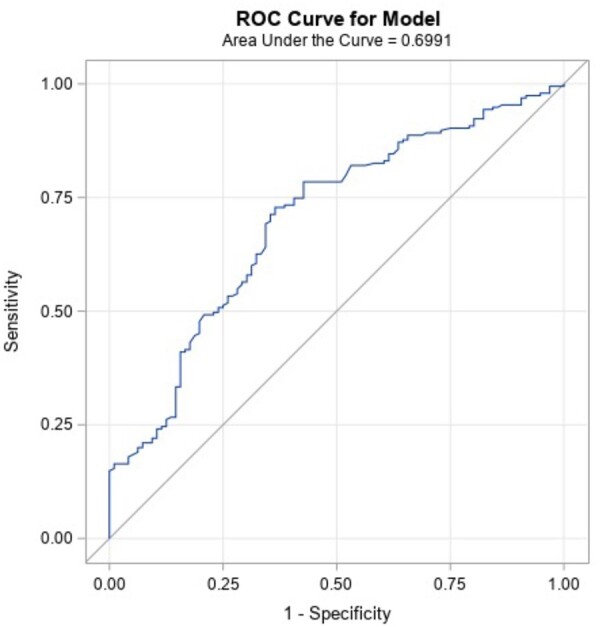
ROC curve of the predictive model (Response Definition 2). The ROC curve for the model fit with Response Definition 2 (≥15-point ODI improvement). ROC curves plot the sensitivity against 1 minus specificity, such that a perfect diagnostic would have an AUC of 1.0 (100%). The AUC for this model is 0.70, for borderline acceptable predictive ability.
Table 7 reports the result of the stepwise logistic regression analyses with the combined response definition ≥50% VAS or ≥15-point ODI improvement. The final logistic regression model included employment status and baseline BDI score. Being employed increased the odds of treatment success, whereas a higher baseline BDI score (greater depression symptoms) decreased the odds of treatment success. The AUC for this model is 0.62, for limited predictive ability. Figure 4 shows the ROC curve for the model.
Table 7.
Predictive model from the final selected model following stepwise logistic regression (Response Definition 3)
| Model | Variable Included | Odds Ratio | P Value | Pseudo R2 | Area Under ROC Curve |
|---|---|---|---|---|---|
|
Baseline ODI | 1.045 | 0.0014 | ||
| 0.06 | 0.64 | ||||
| Baseline BDI | 0.942 | 0.0160 |
Final candidate predictors for the final model are shown: Baseline ODI and baseline BDI score demonstrated a P value <0.05 with Response Definition 3 (≥50% VAS or ≥15-point ODI improvement). Of the variables examined, having a higher baseline ODI (greater functional impact) increased the odds of treatment success, whereas reporting a higher baseline BDI score (greater depression symptoms) decreased the odds of treatment success. The demonstrated ROC curve was 64%, for limited predictive ability.
Figure 4.
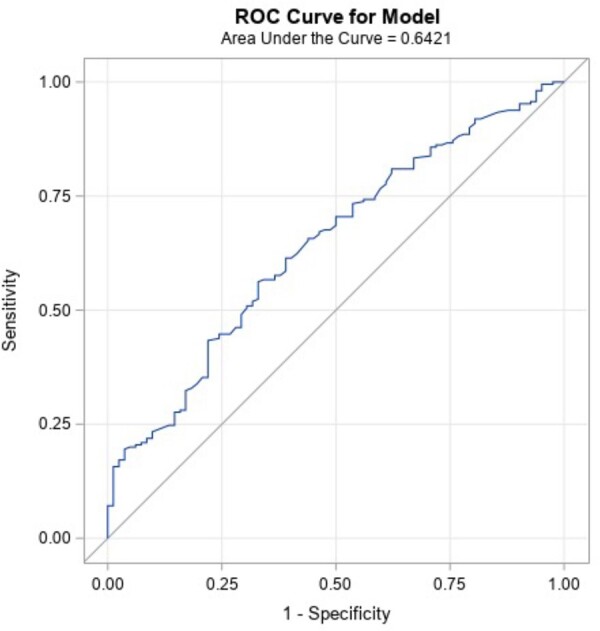
ROC curve of the predictive model (Response Definition 3). The ROC curve for the model fit with Response Definition 3 (≥50% VAS or ≥15-point ODI improvement). ROC curves plot the sensitivity against 1 minus specificity, such that a perfect diagnostic would have an AUC of 1.0 (100%). The AUC for this model is 0.64, for limited predictive ability.
Discussion
This study reports the first analysis of the relationship of demographic and clinical characteristics on treatment success associated with BVN RFA for individuals with chronic, refractory LBP in the context of Type 1 and/or Type 2 Modic changes and a clinical diagnosis of predominant VEP. Pain duration of ≥5 years and a higher baseline ODI score (greater functional disability related to LBP) increased the odds of treatment success. Conversely, baseline opioid use and a higher baseline BDI score (greater depression symptoms) decreased the odds of treatment success based on the three investigated response definitions. A higher BDI score was the only factor that decreased the odds of treatment success for all three responder definitions (≥50% VAS improvement; ≥15-point ODI improvement; and ≥50% VAS or ≥15-point ODI improvement, respectively). However, in all three models, the AUC was 70% or less, indicating limited predictive value.
Previous studies have investigated the relationship of numerous demographic and clinical factors with treatment success of chronic axial LBP. In one large multicenter study of patients who underwent spinal fusion surgery for the treatment of chronic axial LBP, patient characteristics were assessed for predictive capability with a logistic regression model with three individual treatment response definitions (ODI with a 15-point threshold, and back and leg pain numeric rating scale with a 2-point threshold) [41]. That study found that workers’ compensation insurance, being a current or past smoker, asthma, and a lower baseline ODI score were significantly associated with lower odds of functional improvement. Factors that were associated with lower odds of back pain improvement included younger age, nonprivate insurance, current smoking, current spondylolisthesis, use of opiate prescription, and a low baseline back pain numeric rating. In two other studies, age, sex, and duration of symptoms did not demonstrate predictive value for treatment outcomes of spinal fusion [42,43]. Finally, greater patient-reported depression scores appear to be associated with a lesser likelihood of treatment success with spinal fusion [44].
With regard to nonoperative intervention specific to lumbar facet-joint pain causing chronic axial LBP, it has been demonstrated that a successful treatment response to lumbar medial branch radiofrequency ablation (LMB RFA) is not related to sex, body mass index, duration of LBP, or the number of spinal levels denervated [36,37]. Mixed findings have been observed with regard to the relationships of age and baseline opioid use to a successful treatment outcome of LMB RFA [35–37]. Alternatively, the best predictor of response to LMB RFA is the result of diagnostic/prognostic medial branch blocks [45,46].
Although our study demonstrated similar demographic and clinical factors for reduced odds of treatment success, none of those factors were robust predictors and, as such, are not prohibitive when treatment with BVN RFA is considered. Unlike LMB RFA, an appropriate diagnostic/prognostic block is not needed to isolate VEP. It has been hypothesized by some investigators that either provocation or analgesic discography (discoblock) might hold prognostic value for treatment outcomes associated with BVN RFA for VEP, given that it could evoke vertebral endplate deflection [47]. However, this diagnostic technique has intrinsic limitations. VEP could be mediated predominantly by an inflammatory state, with chemical hypersensitivity rather than mechanical force, as suggested by histological research [6]. Consideration of directly blocking the BVN might be a test with prognostic value, but the invasiveness of this diagnostic block compared with other spinal blocks used to determine appropriateness for ablation would be difficult to justify.
The clinical trials conducted to date for patients with chronic LBP used an objective biomarker for VEP (Type 1 or 2 Modic changes) without the presence of meaningful spinal canal or neuroforaminal stenosis, in the context of an overall picture of anterior spinal element pain, and these trials have demonstrated high response rates [19–26,48]. The present analysis further supports using the biomarker of Modic changes within the correct clinical context (similar inclusion/exclusion criteria to the three clinical trials referenced) to identify primary VEP patients who could respond to BVN RFA.
In the present study, the type of Modic changes (Type 1 vs Type 2) was not predictive of treatment response. Investigation of characteristic pain patterns, physical examination findings, and more granular imaging endplate characteristics that might predict treatment success has also been conducted and is reported in a companion publication [49]. Such analyses provide further understanding of bone marrow intensity change characteristics (location on the endplate, area, type of defect, etc.) and whether such characteristics are predictive of treatment success. The ability to discriminate between patients with predominant VEP pain and those with mixed etiologies could provide a more robust and durable treatment response to BVN RFA.
A strength of this analysis is the use of a homogeneous, primary VEP population who were successfully treated as the reference group for identifying response/nonresponse factors. As with all studies, this present investigation contains limitations. Despite a robust retrospective analysis of available demographic and clinical characteristics derived from the prior clinical trials, the potential effect of unknown confounding variables affecting the results cannot be determined. Five subjects were missing ODI or VAS outcomes data and thus could not be included in the analysis. However, the proportion of missing baseline or outcomes variables was small, and we do not believe that this influenced the present findings. Finally, the creation of a more lenient model (lower P value thresholds for inclusion of variables into the predictive model) might have identified more predictive factors. Nevertheless, model thresholds and responder definitions were designed for clinical relevance to support treatment decisions.
Conclusions
This regression analysis of pooled prospective clinical trial cohort data of VEP patients with Type 1 and/or 2 Modic changes did not identify additional meaningful demographic or clinical characteristic predictors for either increased or reduced odds of treatment success of BVN RFA. On the basis of these findings and the high response rates from the three analyzed clinical trials, we recommend that clinicians continue to use objective imaging biomarkers (Type 1 and/or 2 Modic changes), a correlating presentation of anterior spinal element pain, and appropriate clinical judgment to determine optimal candidacy for BVN RFA.
Contributor Information
Barrett S Boody, Indiana Spine Group, Caramel, Indiana.
Beau P Sperry, David Geffen School of Medicine at UCLA, Los Angeles, California.
Katrina Harper, Technomics Research LLC, Minneapolis, Minnesota.
Kevin Macadaeg, Indiana Spine Group, Caramel, Indiana.
Zachary L McCormick, Department of Physical Medicine and Rehabilitation, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Funding sources: An investigator-initiated grant was provided by Relievant Medsystems to support this study.
Disclosures and Conflicts of interest: Barrett S. Boody received investigator research grant funding from Relievant Medsystems Inc. for this study. Zachary L. McCormick has received research funding from Relievant Medsystems Inc., paid directly to the University of Utah. Kevin Macadaeg has received consulting payment by Relievant Medsystems for physician proctoring and procedure training.
Supplement sponsorship: This article appears as part of the supplement entitled “Vertebrogenic Pain and Basivertebral Nerve Radiofrequency Ablation” sponsored by Relievant Medsystems Inc.
References
- 1. Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996–2016. JAMA 2020;323(9):863–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008;299(6):656–64. [DOI] [PubMed] [Google Scholar]
- 3. Keller A, Hayden J, Bombardier C, van Tulder M.. Effect sizes of non-surgical treatments of non-specific low-back pain. Eur Spine J 2007;16(11):1776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fields AJ, Ballatori A, Liebenberg EC, Lotz JC.. Contribution of the endplates to disc degeneration. Curr Mol Biol Rep 2018;4(4):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lotz JC, Fields AJ, Liebenberg EC.. The role of the vertebral end plate in low back pain. Global Spine J 2013;3(3):153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC.. Pathobiology of Modic changes. Eur Spine J 2016;25(11):3723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey JF, Liebenberg E, Degmetich S, Lotz JC.. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat 2011;218(3):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fras C, Kravetz P, Mody DR, Heggeness MH.. Substance P–containing nerves within the human vertebral body. An immunohistochemical study of the basivertebral nerve. Spine J 2003;3(1):63–7. [DOI] [PubMed] [Google Scholar]
- 9. Krug R, Joseph GB, Han M, et al. Associations between vertebral body fat fraction and intervertebral disc biochemical composition as assessed by quantitative MRI. J Magn Reson Imaging 2019;50(4):1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maus TP, Aprill CN.. Lumbar diskogenic pain, provocation diskography, and imaging correlates. Radiol Clin North Am 2012;50(4):681–704. [DOI] [PubMed] [Google Scholar]
- 11. Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: A systematic review and meta-analysis. AJNR Am J Neuroradiol 2015;36(12):2394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen TS, Karppinen J, Sorensen JS, Niinim€aki J, Leboeuf-Yde C.. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with nonspecific low back pain. Eur Spine J 2008;17(11):1407–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bailey JF, Fields AJ, Ballatori A, et al. The relationship between endplate pathology and patient-reported aymptoms for chronic low back pain depends on lumbar paraspinal muscle quality. Spine (Phila Pa 1976) 2019;44(14):1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fields AJ, Ballatori A, Han M, et al. Measurement of vertebral endplate bone marrow lesion (Modic change) composition with water-fat MRI and relationship to patient-reported outcome measures. Eur Spine J 2021;30(9):2549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michalik A, Conger A, Smuck M, Maus TP, McCormick ZL.. Intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebral body endplate low back pain: current evidence and future directions. Pain Med 2021;22(Suppl 1):S24–30. [DOI] [PubMed] [Google Scholar]
- 16. Antonacci MD, Mody DR, Heggeness MH, et al. ; Innervation of the human vertebral body: a histologic study. J Spinal Disord. 1998;11(6):526–31. [PubMed] [Google Scholar]
- 17. Fagan A, Moore R, Vernon-Roberts B, Blumbergs P, Fraser R.. ISSLS prize winner: The innervation of the intervertebral disc: A quantitative analysis. Spine 2003;28(23):2570–6. [DOI] [PubMed] [Google Scholar]
- 18. Degmetich S, Bailey JF, Liebenberg E, Lotz JC.. Neural innervation patterns in the sacral vertebral body. Eur Spine J 2016;25(6):1932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischgrund JS, Rhyne A, Franke J, Sasso R, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: A prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 2018;27(5):1146–56. [DOI] [PubMed] [Google Scholar]
- 20. Khalil J, Smuck M, Koreckij T, et al. A prospective, randomized, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J 2019;19(10):1620–32. [DOI] [PubMed] [Google Scholar]
- 21. Truumees E, Macadaeg K, Pena E, et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Eur Spine J 2019;28(7):1594–602. [DOI] [PubMed] [Google Scholar]
- 22. Smuck M, Khalil JG, Barrett K, et al. A prospective, randomized, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. Reg Anesth Pain Med 2021;46(8):683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macadaeg K, Truumees E, Boody B, et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. NASSJ 2020;3:100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 2-year results from a prospective randomized double-blind sham-controlled multicenter study. Int J Spine Surg 2019;13(2):110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koreckij T, Kreiner S, Khalil JG, et al. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-month treatment arm results. NASSJ 2021;8:100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fischgrund JS, Rhyne A, Macadaeg K, et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5 year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2020;29(8):1925–34. [DOI] [PubMed] [Google Scholar]
- 27. Conger A, Schuster NM, Cheng DS, et al. The effectiveness of intraosseous basivertebral nerve radiofrequency neurotomy for the treatment of chronic low back pain in patients with Modic changes: a systematic review. Pain Med 2021;22(5):1039–54. [DOI] [PubMed] [Google Scholar]
- 28. Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E.. The association between smoking and low back pain: a meta-analysis. Am J Med 2010;123(1):87.e7–e35. [DOI] [PubMed] [Google Scholar]
- 29. Oliveira DS, Vélia Ferreira Mendonça L, Sofia Monteiro Sampaio R, Manuel Pereira Dias de Castro-Lopes J, Ribeiro de Azevedo LF.. The impact of anxiety and depression on the outcomes of chronic low back pain multidisciplinary pain management-a multicenter prospective cohort study in pain clinics with one-year follow-up. Pain Med 2019;20(4): 736–46. [DOI] [PubMed] [Google Scholar]
- 30. Park SM, Kim HJ, Jang S, et al. Depression is closely associated with chronic low back pain in patients over 50 years of age: A cross-sectional study using the Sixth Korea National Health and Nutrition Examination Survey (KNHANES VI-2). Spine (Phila Pa 1976) 2018;43(18): 1281–8. [DOI] [PubMed] [Google Scholar]
- 31. Dario AB, Ferreira ML, Refshauge KM, et al. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. Spine J 2015;15(5): 1106–17. [DOI] [PubMed] [Google Scholar]
- 32. McCormick Z, Cushman D, Casey E, et al. Factors associated with pain reduction after transforaminal epidural steroid injection for lumbosacral radicular pain. Arch Phys Med Rehabil 2014;95(12):2350–6. PMID: 25108099 [DOI] [PubMed] [Google Scholar]
- 33. Ghahreman A, Bogduk N.. Predictors of a favorable response to transforaminal injection of steroids in patients with lumbar radicular pain due to disc herniation. Pain Med 2011;12(6):871–9. [DOI] [PubMed] [Google Scholar]
- 34. Inman SL, Faut-Callahan M, Swanson BA, Fillingim RB.. Sex differences in responses to epidural steroid injection for low back pain. J Pain 2004;5(8):450–7. [DOI] [PubMed] [Google Scholar]
- 35. Conger A, Burnham T, Salazar F, et al. The effectiveness of radiofrequency ablation of medial branch nerves for chronic lumbar facet joint syndrome in patients selected by guideline-concordant dual comparative medial branch blocks. Pain Med 2020;21(5):902–9. [DOI] [PubMed] [Google Scholar]
- 36. Yadav A, Hagedorn JM, D'Souza RS, Engle AM, Deer TR.. effect of patient characteristics on reported outcomes over 12 months following lumbar radiofrequency ablation: a retrospective review of 500 patients. Pain Pract 2021;21(2):152–9. [DOI] [PubMed] [Google Scholar]
- 37. Cohen SP, Hurley RW, Christo PJ, et al. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain 2007;23(1):45–52. [DOI] [PubMed] [Google Scholar]
- 38. Bogduk N, Kennedy DJ, Vorobeychik Y, Engel A.. Guidelines for composing and assessing a paper on treatment of pain. Pain Med 2017;18(11):2096–104. [DOI] [PubMed] [Google Scholar]
- 39. McCormick ZL, Walega DR.. Managing patient expectations is vital to successful pain management. Pain Med 2019;20(7):1453–4. [DOI] [PubMed] [Google Scholar]
- 40. Mandrekar J. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010;5(9):1315–6. [DOI] [PubMed] [Google Scholar]
- 41. Khor S, Lavallee D, Cizik AM, et al. Development and validation of a prediction model for pain and functional outcomes after lumbar spine surgery. JAMA Surg 2018;153(7):634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohtori S, Orita S, Yamauchi K, et al. Do physical symptoms predict the outcome of surgical fusion in patients with discogenic low back pain? Asian Spine J 2016;10(3):509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marshman LA, Kasis A, Krishna M, Bhatia CK.. Does symptom duration correlate negatively with outcome after posterior lumbar interbody fusion for chronic low back pain? Spine (Phila Pa 1976) 2010;35(6):657–65. [DOI] [PubMed] [Google Scholar]
- 44. Daubs MD, Norvell DC, McGuire R, et al. Fusion versus nonoperative care for chronic low back pain: Do psychological factors affect outcomes? Spine (Phila Pa 1976). 2011;36(Suppl 21):S96–109. [DOI] [PubMed] [Google Scholar]
- 45. Vorobeychik Y, Stojanovic MP, McCormick ZL.. Radiofrequency denervation for chronic low back pain. JAMA 2017;318(22):2254–5. [DOI] [PubMed] [Google Scholar]
- 46. McCormick ZL, Vorobeychik Y, Gill JS, et al. Guidelines for composing and assessing a paper on the treatment of pain: a practical application of evidence-based medicine principles to the Mint randomized clinical trials. Pain Med 2018;19(11):2127–37. [DOI] [PubMed] [Google Scholar]
- 47. Heggeness MH, Doherty BJ.. Discography causes end plate deflection. Spine (Phila Pa 1976) 1993;18(8):1050–3. [DOI] [PubMed] [Google Scholar]
- 48. De Vivo AE, D'Agostino G, D'Anna G, et al. Intra-osseous basivertebral nerve radiofrequency ablation (BVA) for the treatment of vertebrogenic chronic low back pain. Neuroradiology 2021;63(5):809–15. [DOI] [PubMed] [Google Scholar]
- 49. McCormick Z, Conger A, Smuck M, et al. Magnetic resonance imaging characteristics associated with treatment success from basivertebral nerve ablation: an aggregated cohort study of multicenter prospective clinical trials data. Pain Med 2022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]