Figure 6. 12/15-LOX signaling is linked to activation of the integrated stress response and relative suppression of PD-L1 expression in islets.
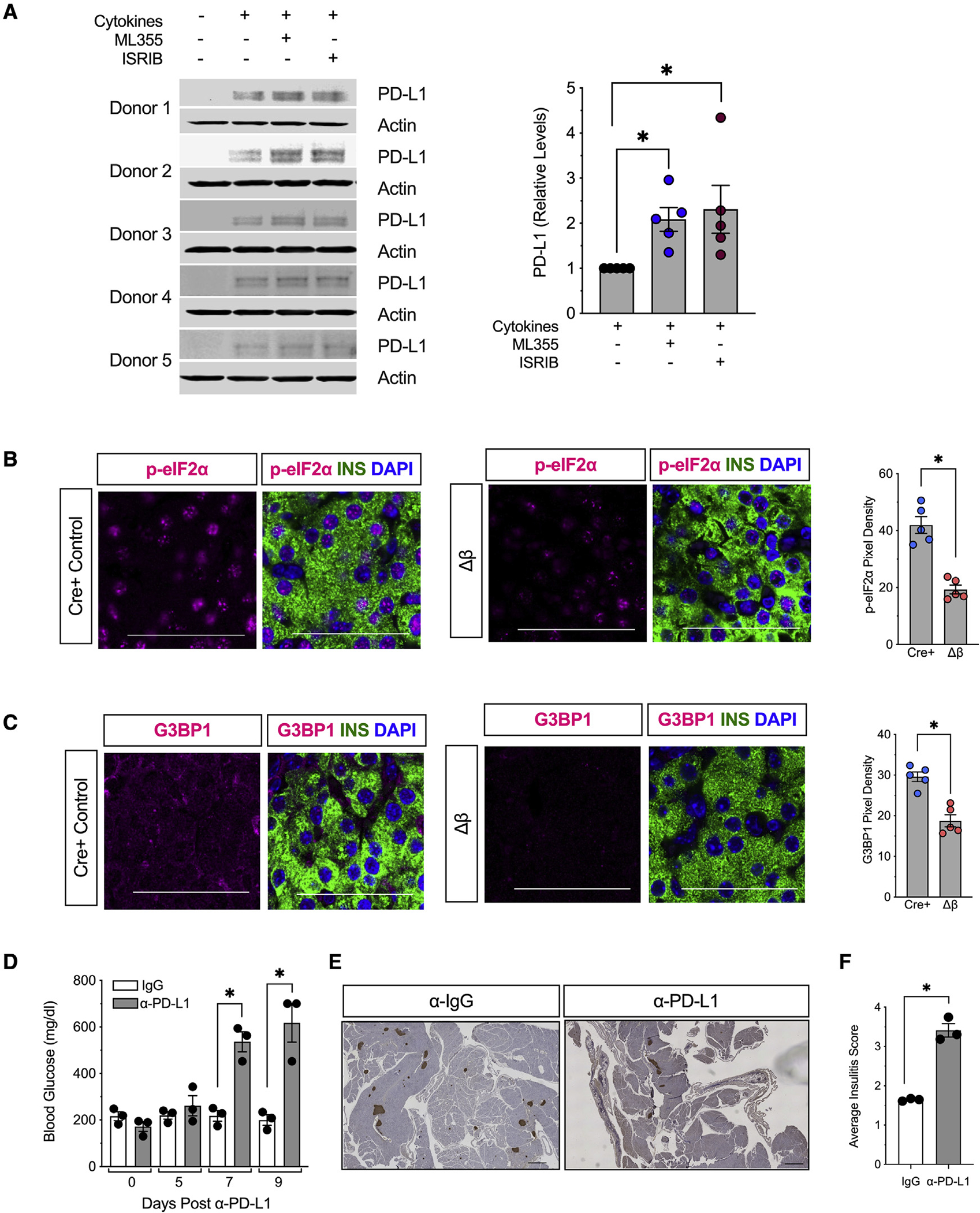
(A) Isolated human islets (N = 5 different donors) were treated with a mixture of proinflammatory cytokines (IL-1β and IFN-γ) for 24 h in the presence of the human 12-LOX inhibitor ML-355 prior to protein isolation. Individual immunoblots (left) and quantification of protein for PD-L1 (right).
(B) Representative images of pancreata from 8-week-old mice for phospho-eIF2α (magenta), β cells (insulin, green), and nuclei (DAPI, blue), and quantification of phospho-eIF2α pixel density from islets (from N = 5 mice). Scale bars, 50 μm.
(C) Representative images of pancreata from 8-week-old NOD-Cre+ and NOD-Δβ immunostained for G3BP1 (magenta), β cells (insulin, green), and nuclei (DAPI, blue), and quantification of G3BP1 pixel density from islets (from N = 5 mice). Scale bars, 50 μm.
(D–F) Female NOD-Δβ mice were administered a single dose of anti-PD-L1 monoclonal antibody (α-PD-L1) (N = 3 mice) or IgG isotype control (N = 3 mice) intraperitoneally.
(D) Non-fasting blood glucose levels.
(E) Images of whole pancreatic sections from representative mice at 9 days post α-PD-L1 or IgG administration immunostained for β cells (insulin, brown) and counterstained with hematoxylin. Scale bars, 500 μm.
(F) Average insulitis score (from N = 3 mice).
Data are expressed as the mean ± SEM. *p < 0.05.
