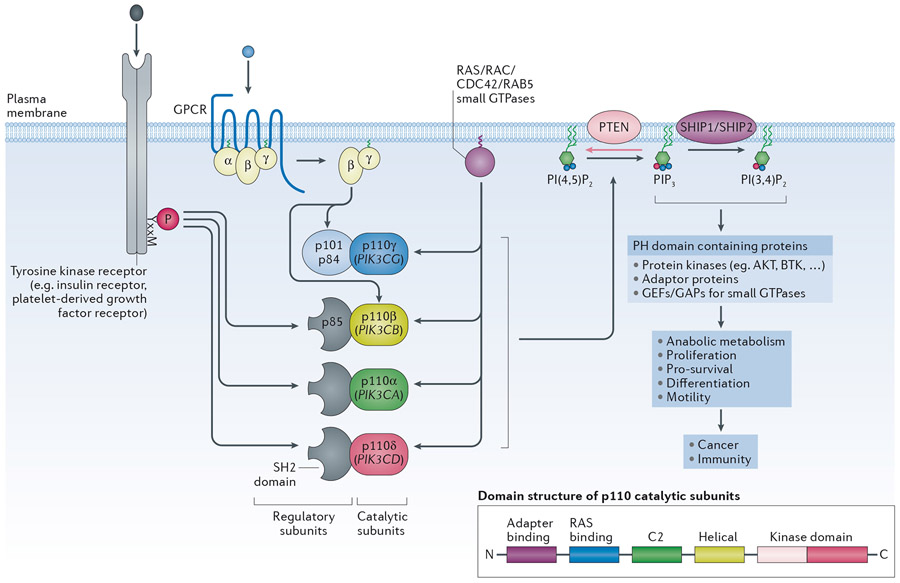
Figure 1 – General overview of signalling by class I PI3K isoforms.
The class IA PI3K catalytic subunits (p110α, β and δ) bind the p85 regulatory subunits which keep the p85/p110 complex in an inactive, cytosolic form. The p85 subunits have two SH2 domains that allow the p85/p110 heterodimers to bind to phosphorylated tyrosine residues in membrane-associated proteins, such as receptors and adaptor proteins, thereby recruiting the PI3K heterodimer to its lipid substrates while simultaneously disinhibiting its enzymatic activity. Mammals have three genes for p85 regulatory subunits, namely PIK3R1 (encoding p85α, p55α and p50α), PIK3R2 (encoding p85β) and PIK3R3 (encoding p55γ). p110γ, the sole member of the class IB PI3Ks, binds p101/p84 regulatory subunits which do not have homology to p85 or other proteins, and which permit p110γ to engage with Gβγ subunits downstream of GPCRs. Class I PI3Ks can also engage with small GTPases such as members of the Ras (p110α, p110δ, p110γ) or Cdc42, Rac or Rab5 families (p110β). Unlike PI3Kα and PI3Kδ, PI3Kβ is also activated by Gβγ subunits downstream of GPCRs and appears to require more inputs to become fully activated compared to PI3Kα. (Insert): overall domain structure of the p110 catalytic subunits.
Class I PI3Ks phosphorylate the 3-position of the inositol ring of a specific phosphatidylinositol (PtdIns) lipid, namely phosphatidylinositol-(4,5)-bisphosphate (PtdIns(4,5)P2), converting it to phosphatidylinositol-(3,4,5)-trisphosphate (PtdIns(3,4,5)P3, or PIP3). PIP3 can be converted to PtdIns(3,4)P2 following dephosphorylation of the 5’-position by the 5-phosphatases SHIP1 and SHIP2. Together, PIP3 and PtdIns(3,4)P2 function as second messengers downstream of class I PI3Ks by interacting with 3-phosphoinositide-binding pleckstrin homology (PH) domains found in diverse proteins, including protein kinases (AKT, BTK), adaptor proteins and regulators of small GTPases. The tumour suppressor phosphatase and tensin homolog (PTEN) 3-phosphoinositide phosphatase dampens class I PI3K signalling, by dephosphorylating PIP3 and PtdIns(3,4)P2. PTEN is frequently somatically inactivated in cancer, through a wide range of mechanisms, including loss-of-expression and/or mutation. PTEN inactivation is also the cause of a developmental syndrome known as PTEN Hamartoma Tumour Syndrome (PHTS) in which one gene copy of PTEN has been partially or fully inactivated. Individuals with PHTS are predisposed to benign overgrowths, neurodevelopmental abnormalities as well as specific cancers in adulthood.