Summary
Contemporary diagnosed WM patients, compared to the general population, continue to experience excess mortality regardless of having survived up to 15 years post‐diagnosis. This gradual increase in excess mortality might result from the incurable nature of this disease characterized by multiple relapses throughout the disease course with limited efficacious treatment options in the released/refractory setting.
Keywords: lymphoplasmacytic lymphoma, Waldenström’s macroglobulinaemia, conditional relative survival, population‐based
Introduction
Lymphoplasmacytic lymphoma (LPL) is a rare, indolent B‐cell non‐Hodgkin lymphoma predominantly involving the bone marrow (BM). Increased serum levels of monoclonal immunoglobulin M (IgM) are present in most LPL patients, defined as Waldenström’s macroglobulinaemia (WM). 1 Lymphoplasmacytic lymphoma without IgM and WM is hereafter referred to as LPL/WM.
The few available population‐based studies in LPL/WM revealed that the introduction of rituximab to the therapeutic arsenal of LPL/WM improved the population‐level survival of LPL/WM patients. 2 , 3 , 4 , 5 , 6 , 7 These studies present survival estimates measured from diagnosis, which is helpful to inform on the prognosis for newly diagnosed patients. However, these estimates can be somewhat discouraging due to patients who die within the first years post diagnosis. Conditional relative survival (CRS) is a concept that refers to the likelihood of survival — corrected for the life expectancy in the general population — after having survived from a specified time since diagnosis. Generally, CRS tends to increase with increasing years already survived since there are more ‘cured’ patients. CRS is a practical approach as it provides survivors with information on prognosis improvements at various stages of survivorship.
To fill the knowledge gap and inform long‐term LPL/WM survivors about their CRS, this nationwide population‐based study examined up‐to‐date estimates of five‐year relative survival (RS) at diagnosis and for each additional year survived up to 15 years post diagnosis among LPL/WM patients diagnosed in the Netherlands.
The nationwide Netherlands Cancer Registry (NCR) covers >95% of all newly diagnosed malignancies in The Netherlands since 1989. The NCR relies on case ascertainment via the Nationwide Histopathology and Cytopathology Data Network and the Nationwide Registry of Hospital Discharges (i.e. inpatient and outpatient discharges).
All patients diagnosed with BM‐confirmed LPL/WM between 1989 and 2018 — with follow‐up for survival until 31 December 2019 — were selected from the NCR using International Classification of Diseases for Oncology morphology codes 9671 and 9761. Data on the dates of birth, diagnosis, and last known vital status (i.e. alive, death or emigration) and sex were available for individual patients. Twenty‐eight LPL/WM cases diagnosed through autopsy were excluded. The Privacy Review Board of the NCR approved the use of anonymous data for this study.
Relative survival was calculated to estimate disease‐specific survival in the absence of information on the cause of death. 8 Relative survival is defined as the ratio of observed to expected survival, of which the latter is estimated using national annual life tables and matched to the patient by age, sex, and calendar year. We calculated five‐year RS at diagnosis and five‐year CRS for each additional year survived up to fifteen years after diagnosis, conditional on being alive at the beginning of that year. The Ederer II method was used to estimate expected survival. 9
The empirically validated hybrid approach was used to generate up‐to‐date survival probabilities for patients diagnosed in the period 1989–2018 who were alive between the follow‐up interval of 2008–2019. 10 This approach is suitable for predicting up‐to‐date estimates of RS when incidence data lag behind mortality data. Our settings used for the hybrid approach resulted in 20 years of post‐diagnostic follow‐up information to compute five‐year CRS up to 15 years post diagnosis. The survival probabilities of this study can be interpreted as the predicted survival probabilities for patients diagnosed in the period 2008–2019. The statistical analysis and how the survival data were structured under the hybrid approach is extensively described in the supporting information.
Survival statistics were computed for the overall cohort and according to sex and age at diagnosis (≤65 and >65 years) with associated 95% confidence intervals (CIs) and standard errors (SEs). The age groups were chosen based on the age cut‐off of the International Prognostic Scoring System for WM. There is minimal excess mortality compared to the general population when five‐year RS exceeds 95%. 11 Survival differences between the point estimates were assessed by 95% CI overlap. Only survival estimates with a SE of ≤5% were presented. This choice relates to the precision of the survival estimates. STATA/SE 16.1 (StataCorp LP, College Station, TX, USA) was used for the analyses.
A total of 6 234 LPL/WM patients (median age, 70 years; and 61% males) were diagnosed in The Netherlands in the period 1989–2018 (Table I). The number of patients at risk under the hybrid approach and estimates of five‐year CRS at diagnosis and 5‐, 10‐, and 1five‐years post diagnosis are presented in Table I, stratified by sex and age at diagnosis. A graphical representation of five‐year CRS up to fifteen years post diagnosis for the overall cohort and according to sex and age at diagnosis is presented in Fig 1. The overall five‐year RS measured from diagnosis was 86% (95% CI, 84–88%) and gradually decreased with additional years survived to 75% (95% CI, 68–82%) at 15 years post diagnosis (Fig 1A). This trend was emulated according to sex, with no sex differential in survival over time (Fig 1B). However, there was an age differential in five‐year RS at diagnosis that essentially persisted with each year survived post diagnosis (Table I and Fig 1C). Also, five‐year CRS decreased with each additional year survived across the two age groups. However, for patients aged ≤65 years, the excess mortality remains nearly constant at approximately 20% after ten years post diagnosis.
Table I.
Conditional five‐year relative survival among patients with LPL/WM at diagnosis, after 5 and 10 years post‐diagnosis.
| Characteristics | No. of patients at diagnosis | No. of patients at risk under the hybrid approach after x years | Conditional five‐year relative survival (95% CI) | Reliable estimates up to x years* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | 0 | 5 | 10 | 15 | At diagnosis | At 5 years | At 10 years | At 15 years | ||
| Total no. of patients | 6 234 | (100) | 4 296 | 2 424 | 1 154 | 478 | 86 (84–88) | 84 (81–87) | 77 (73–82) | 75 (68–82) | 15 |
| Sex | |||||||||||
| Male | 3 782 | (61%) | 2639 | 1421 | 626 | 243 | 85 (83–88) | 83 (79–87) | 76 (71–83) | 72 (64–84) | 14 |
| Female | 2 452 | (39%) | 1657 | 1003 | 528 | 235 | 87 (84–90) | 85 (81–90) | 79 (74–86) | 77 (68–87) | 15 |
| Age at diagnosis | |||||||||||
| ≤65 years | 2 204 | (35%) | 1729 | 1207 | 716 | 360 | 93 (91–95) | 90 (87–93) | 82 (78–87) | 80 (74–87) | 15 |
| >65 years | 4 030 | (65%) | 2567 | 1217 | 438 | 118 | 82 (79–85) | 77 (72–82) | 69 (60–79) | –† | 10 |
CRS estimates are reliable when the standard error of the estimate is 5% or below.
Standard error of the CRS estimate is above 5%.
Fig 1.
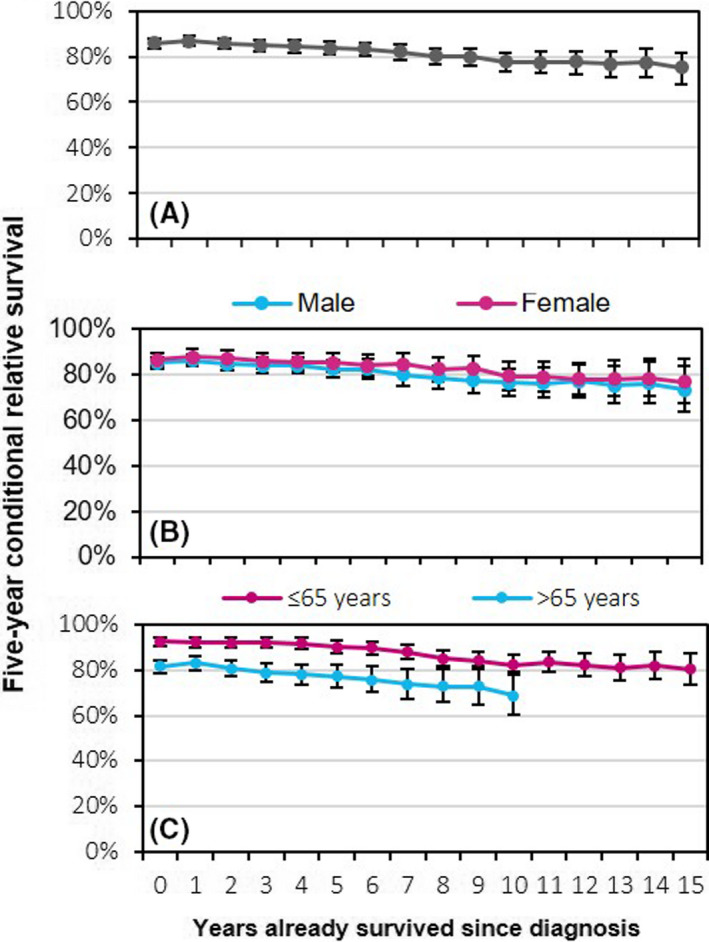
Five‐year conditional relative survival (CRS) up to fifteen years post diagnosis among patients diagnosed with lymphoplasmacytic lymphoma/Waldenström's macroglobulinaemia in The Netherlands, 1989–2018. The five‐year CRS is presented for the overall cohort (A) and according to sex (B), and age at diagnosis (C). The error bars for the point estimates indicate 95% confidence intervals. [Colour figure can be viewed at wileyonlinelibrary.com]
This is the first population‐based study assessing CRS in LPL/WM. This study provides a better understanding of the prognosis of LPL/WM patients with each additional year survived post diagnosis.
The introduction of rituximab in the early–mid‐2000s changed the treatment paradigm of LPL/WM. It essentially replaced the use of single‐ or multiagent chemotherapy. Rituximab or rituximab‐based regimens [e.g. with chemotherapy, a proteasome inhibitor, or a Bruton's tyrosine kinase (BTK) inhibitor] have the most prominent position in contemporary treatment protocols for LPL/WM patients. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Despite these advances, we showed that LPL/WM patients across all age groups continue to experience excess mortality throughout the 20 years of post‐diagnostic follow‐up in the rituximab era. Although five‐year RS at diagnosis among patients aged ≤65 and >65 years at diagnosis is relatively high — especially among patients aged ≤65 years — it is somewhat disheartening that the age difference in survival at diagnosis persisted over time and excess mortality remains to increase regardless of having survived several years post diagnosis. The gradual increase in excess mortality with each additional year survived post diagnosis might result from the incurable nature of LPL/WM, characterized by multiple relapses throughout the disease course with limited efficacious treatment options in the released/refractory setting. Also, treatment‐related sequelae, transformation to a high‐grade lymphoma, the development of secondary primary malignancies, comorbid conditions at diagnosis or acquired post diagnosis, and infections might attribute to excess mortality.
The strength of this study is the use of a nationwide population‐based cancer registry providing data to estimate long‐term and up‐to‐date survival expectations in the general LPL/WM population. Limitations of this study include the lack of detailed data on prognostic indices and therapy throughout most of the registry to stratify the survival estimates. Also, information on the cause of death was lacking.
Despite these limitations, this study highlights that contemporary diagnosed LPL/WM patients, compared to the general population, continue to experience considerable excess mortality with each additional year survived post diagnosis. CRS estimates offer LPL/WM patients information about their survival expectations with each year survived post diagnosis and provides means to physicians to tailor surveillance and follow‐up activities. Our study results touch upon the need for novel curative treatment strategies to reduce short‐ and long‐term excess mortality in LPL/WM patients.
Author contributions
AGD, KA and MJK designed the study; KA analyzed the data; AGD provided statistical support; OV was responsible for the collected data; KA wrote the manuscript with contributions from all authors, who also interpreted the data, and read, commented on, and approved the final version of the manuscript.
Conflicts of interest
There is no financial support for this work that could have influenced the outcomes described in the manuscript. MJK has received research support from Roche and Takeda and has received honoraria for advisory boards or presentations from Roche and Takeda. All remaining authors declare to have no competing financial interests.
Supporting information
Supplemental Table 1. Period of diagnosis considered for the calculation of the conditional relative survival at diagnosis and after one through fifteen years post‐diagnosis.
Supplemental Figure 1. Structure of the survival data used under the hybrid approach.
Supplemental Figure 2. Structure of the survival data used in the computation of 5‐year relative survival at diagnosis (A) and after five (B), ten (C), and fifteen (D) years post‐diagnosis.
Acknowledgements
The authors would like to thank the registration clerks of the Netherlands Cancer Registry (NCR) for their dedicated data collection. The nationwide population‐based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation (IKNL).
References
- 1. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sekhar J, Sanfilippo K, Zhang Q, Trinkaus K, Vij R, Morgensztern D. Waldenström macroglobulinemia: a surveillance, epidemiology, and end results database review from 1988 to 2005. Leuk Lymphoma. 2012;53:1625–6. [DOI] [PubMed] [Google Scholar]
- 3. Castillo JJ, Olszewski AJ, Cronin AM, Hunter ZR, Treon SP. Survival trends in Waldenström macroglobulinemia: an analysis of the surveillance, epidemiology and end results database. Blood. 2014;123:3999–4000. [DOI] [PubMed] [Google Scholar]
- 4. Kristinsson SY, Eloranta S, Dickman PW, Andersson T‐L, Turesson I, Landgren O, et al. Patterns of survival in lymphoplasmacytic lymphoma/Waldenström macroglobulinemia: a population‐based study of 1,555 patients diagnosed in Sweden from 1980 to 2005. Am J Hematol. 2013;88:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groves FD, Travis LB, Devesa SS, Ries LA, Fraumeni JF Jr. Waldenström's macroglobulinemia: incidence patterns in the United States, 1988–1994. Cancer. 1998;82:1078–81. [PubMed] [Google Scholar]
- 6. Jeong S, Kong SG, Kim DJ, Lee S, Lee HS. Incidence, prevalence, mortality, and causes of death in Waldenström macroglobulinemia: a nationwide, population‐based cohort study. BMC Cancer. 2020;20:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kastritis E, Kyrtsonis M‐C, Hatjiharissi E, Symeonidis A, Michalis E, Repoussis P, et al. No significant improvement in the outcome of patients with Waldenström's macroglobulinemia treated over the last 25 years. Am J Hematol. 2011;86:479–83. [DOI] [PubMed] [Google Scholar]
- 8. Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–17. [DOI] [PubMed] [Google Scholar]
- 9. Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–21. [PubMed] [Google Scholar]
- 10. Brenner H, Rachet B. Hybrid analysis for up‐to‐date long‐term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer. 2004;40:2494–501. [DOI] [PubMed] [Google Scholar]
- 11. Janssen‐Heijnen MLG, Gondos A, Bray F, Hakulinen T, Brewster DH, Brenner H, et al. Clinical relevance of conditional survival of cancer patients in europe: age‐specific analyses of 13 cancers. J Clin Oncol. 2010;28:2520–8. [DOI] [PubMed] [Google Scholar]
- 12. Kyle RA, Greipp PR, Gertz MA, Witzig TE, Lust JA, Lacy MQ, et al. Waldenström's macroglobulinaemia: a prospective study comparing daily with intermittent oral chlorambucil. Br J Haematol. 2000;108:737–42. [DOI] [PubMed] [Google Scholar]
- 13. Leblond V, Johnson S, Chevret S, Copplestone A, Rule S, Tournilhac O, et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol. 2013;31:301–7. [DOI] [PubMed] [Google Scholar]
- 14. Treon SP, Emmanouilides C, Kimby E, Kelliher A, Preffer F, Branagan AR, et al. Extended rituximab therapy in Waldenström's macroglobulinemia. Ann Oncol. 2005;16:132–8. [DOI] [PubMed] [Google Scholar]
- 15. Santos‐Lozano A, Morales‐Gonzalez A, Sanchis‐Gomar F, Cristi‐Montero C, Fiuza‐Luces C, Pareja‐Galeano H, et al. Response rate to the treatment of Waldenström macroglobulinemia: a meta‐analysis of the results of clinical trials. Crit Rev Oncol Hematol. 2016;105:118–26. [DOI] [PubMed] [Google Scholar]
- 16. Dimopoulos MA, Anagnostopoulos A, Kyrtsonis M‐C, Zervas K, Tsatalas C, Kokkinis G, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25:3344–9. [DOI] [PubMed] [Google Scholar]
- 17. Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: an open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet. 2013;381:1203–10. [DOI] [PubMed] [Google Scholar]
- 18. Dimopoulos MA, Tedeschi A, Trotman J, García‐Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Engl J Med. 2018;378:2399–410. [DOI] [PubMed] [Google Scholar]
- 19. Gertz MA. Waldenström macroglobulinemia treatment algorithm 2018. Blood Cancer J. 2018;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Period of diagnosis considered for the calculation of the conditional relative survival at diagnosis and after one through fifteen years post‐diagnosis.
Supplemental Figure 1. Structure of the survival data used under the hybrid approach.
Supplemental Figure 2. Structure of the survival data used in the computation of 5‐year relative survival at diagnosis (A) and after five (B), ten (C), and fifteen (D) years post‐diagnosis.


