Abstract
Background
Direct oral anticoagulants (DOACs) do not require concentration monitoring. However, whether DOAC concentrations are stable and their variation between and within patients is not well studied.
Methods
Patients on vitamin K antagonists (VKA) who switched to rivaroxaban, apixaban, or dabigatran were included between 2018 and 2020. Blood was drawn at DOAC trough and peak concentrations at week 0, 2, and 8. Plasma drug concentrations were determined by anti‐factor Xa concentrations (rivaroxaban, apixaban) or diluted thrombin time (dabigatran). Inter‐ and intra‐individual variability was assessed by calculating the coefficient of variation (CV). Linear regression models were employed to evaluate associations between DOAC trough concentrations and previous VKA dosage, creatinine clearance, and body mass index (BMI).
Results
One hundred fifty‐two patients were included, of whom 96 (63%) were male and with a mean age of 73.9 ± 8.4 years. For the inter‐individual variability, the CV ranged between 48% and 81% for trough values and between 25% and 69% for peak values among patients using the recommended DOAC dose. Intra‐individual variability was substantially lower, as here the CV ranged between 18% and 33% for trough values and between 15% and 29% for peak values among patients using the recommended DOAC dose. Previous VKA dosage and creatinine clearance were inversely associated with DOAC trough concentrations. No association was found between BMI and DOAC trough concentrations.
Conclusion
Inter‐individual variability of DOAC concentrations was higher than intra‐individual variability. Lower previous VKA dosage and creatinine clearance were associated with higher DOAC trough concentrations. These findings support further study into an optimal target range, in which the risks of both bleeding and thrombosis are minimal.
Keywords: anticoagulants, blood coagulation test, direct thrombin inhibitors, direct‐acting oral anticoagulant, factor Xa inhibitor
Essentials.
It is not well known whether direct oral anticoagulant (DOAC) concentrations are stable over time within and between patients.
DOAC peak and trough concentrations were determined for 152 patients at three different time points.
Inter‐individual variability was substantial and higher than intra‐individual variability.
These findings support further study into an optimal target range for DOACs.
1. INTRODUCTION
The number of direct oral anticoagulant (DOAC) users for prevention of arterial thromboembolism in non‐valvular atrial fibrillation (NVAF) or management of venous thromboembolism (VTE) has been steadily rising over the last decade, 1 thereby at least partly replacing the use of vitamin K antagonists (VKA) for these indications. 2 In large randomized clinical trials and observational studies, DOACs were found to be non‐inferior to VKAs in terms of clinical outcomes (bleeding, thrombosis). 3 , 4 , 5 , 6 In contrast to treatment with VKAs, which requires both initial dose adjustments to achieve the optimal effect on blood clotting (i.e., titration) and continued monitoring due to a variation in the drug’s effect on coagulation over time, 7 treatment with DOACs currently does not require titration or monitoring in clinical practice. Though this is perceived as an advantage of DOACs over VKAs, it is doubtful whether pharmacokinetic profiles of DOACs in clinical settings are indeed as stable as expected. 8 , 9 , 10 Variation in drug concentrations for any anticoagulant may not be desirable, as achieving optimal balance between bleeding and clotting is important to prevent adverse events. 11 So far, the therapeutic ranges where this is the case for DOAC drug concentrations are not defined. Several studies, however, provide clues for the existence of a therapeutic window for DOACs (i.e., higher DOAC concentrations increase the bleeding risk while lower DOAC concentrations increase the risk of thromboembolic events). 12 , 13 , 14 , 15 To establish whether measuring DOAC concentrations may improve patient safety, it is crucial to know whether patients have stable DOAC drug concentrations over time. Currently, knowledge on the stability of pharmacokinetics of DOACs and variation between patients and especially within patients over time, is limited. In addition, little is known about which factors influence DOAC trough concentrations. In the current KIDOAC (Pharamcokinetics of Direct Oral Anticoagulants) study, we therefore aimed to determine the inter‐ and intra‐individual variability of drug concentrations in patients treated with DOACs in daily practice.
2. METHODS
2.1. Design, study population, and medication
A follow‐up study was conducted that included patients who recently started DOAC of whom DOAC concentrations were measured at several predefined time points. Electronic health records of three anticoagulation clinics in the Netherlands (located in Leiden, The Hague, and Amsterdam), containing information on all VKA‐treated patients in those regions, were searched every week between March 2018 and March 2020 for patients who were recently switched from VKA to DOAC by their treating physician (either their cardiologists, internists, or general practitioners). These patients were screened for eligibility to participate in the KIDOAC study. Inclusion criteria were: age 18 years or over; the DOAC they were switched to was either rivaroxaban, apixaban, or dabigatran; and provision of informed consent before any study‐specific procedure. Exclusion criteria were: life expectancy less than 6 months; serious or unstable medical or psychological conditions that, in the opinion of the investigators, would compromise the person’s successful participation in the study (including alcohol or drug abuse); and previous participation in the KIDOAC study. The dosages of rivaroxaban, apixaban, and dabigatran used by study patients were prescribed by their treating physician and were based on those recommended for NVAF and VTE: rivaroxaban 20 mg once daily (or 10 mg or 15 mg once daily if reduced dose was prescribed), apixaban 5 mg twice daily (or 2.5 mg twice daily if reduced dose was prescribed), and dabigatran 150 mg twice daily (or 110 mg twice daily if reduced dose was prescribed). After screening for eligibility, patients were asked for participation and scheduled for blood sampling procedures. The first study appointment was within 2 months after the date of switching from VKA to DOAC.
2.2. Study procedures and blood sampling
Study patients were planned to be seen for blood sampling on three different days (at day 0, 2 weeks after day 0, and 8 weeks after day 0). The total follow‐up period was 8 weeks from the first visit onward. Depending on their preferences, patients visited the anticoagulation clinics or received research nurses at home for blood sampling, and were asked to keep their normal routine in DOAC intake. During each visit, the following information was collected or verified: age, sex, DOAC dose and anticoagulation indication (all indications were counted separately), adherence to DOAC (defined as ever forgetting to take the DOAC during the follow‐up period), comorbidity and disease history, comedication, height and weight, exposure to cardiovascular risk factors (e.g., hypertension, diabetes mellitus, smoking history), and previous VKA dose (which was derived from electronic patient files from the anticoagulation clinic and was defined as the last known acenocoumarol or phenprocoumon mean daily dose before switching to DOAC). On each day of a study visit, blood sampling was performed at two separate time points through venipuncture. The first sampling was done before DOAC intake on the day of the sampling and ±12 h (apixaban/dabigatran) or ±24 h (rivaroxaban) after the last DOAC dose intake the day before (C‐trough), and the second sampling was performed ±2 h after the DOAC intake on the day of the blood sampling (C‐max). 16 , 17 The timing of drug intake and blood withdrawal was accurately recorded for each visit. At each sampling, one sodium citrate 3.2% tube (5 mL) and two serum tubes (3 mL and 5 mL) were collected. All blood tubes were centrifuged at 2500 g for 10 min at 18°C and samples were stored at −80°C within 4 h after collection until analysis.
2.3. Study parameters
The ACL‐TOP‐700 analyzer (Werfen) was used to measure the prothrombin time (PT, HemosIL ReadiPlasTin) and rivaroxaban or apixaban calibrated anti‐factor Xa assays (HemosIL, Liquid anti‐Xa). 18 , 19 The same analyzer was used to measure plasma drug concentrations for dabigatran using the diluted thrombin time (dTT, HemosIL Direct thrombin inhibitor) calibrated with a dabigatran calibrator 20 and the activated partial thromboplastin time (APTT, HemosIL Synthasil). The measurements were carried out following the manufacturer’s instructions. To minimize the risk of laboratory variation, all DOAC measurements were performed on the same day, using reagents from the same batch. Outlying results (i.e., values outside the two times standard deviation [SD] range, or peak values that were only slightly higher than the trough values) were remeasured in duplicate. In addition, creatinine levels were measured (Roche, COBAS 8000 modular) after which the creatinine clearance (CrCl) was estimated using the Cockcroft‐Gault formula. 21
2.4. Ethical considerations
The study was approved by the Medical Ethics Committee of the Leiden University Medical Center and was in accordance with the Declaration of Helsinki and guidelines for good clinical practice. Each study participant provided a written informed consent document. Participants received financial compensation for travel costs if indicated.
2.5. Sample size considerations
A previous study (on rivaroxaban) provided a standard deviation of the C‐max between patients of 30 μg/L. 22 Based on a 2‐sided alpha of 0.05 and 80% power, a minimum difference of 20 μg/L in C‐max within and between patients can be detected when 36 patients per DOAC are included. We aimed to include 50 patients per DOAC type, to account for any loss to follow‐up and to have more power for subgroup‐ or restriction analyses.
2.6. Statistical analysis
For the baseline characteristics, continuous variables were presented as means ± SD and categorical variables were presented as numbers and percentages, stratified by DOAC type. Inter‐individual variability was assessed for each DOAC by calculating the mean values and ranges (min‐max) at each time point, both for trough and peak concentrations. In addition, the coefficient of variation (CV), which was calculated using the formula (SD/mean) x 100, was provided for peak and trough concentrations for each DOAC. Intra‐individual variability was assessed by calculating the CV for all available trough and peak concentrations for each patient, for which a minimum of two trough or peak concentrations needed to be available to be included in the analysis. The averages of the individual CVs were presented for each DOAC. The 20th and 80th percentile of trough and peak concentrations and the number of patients whose concentrations were outside these ranges were calculated to get more insight into whether patients tended to have outlying DOAC concentrations only once or multiple times, in alignment with previous studies on DOAC concentrations. 8 , 9 In an explanatory analysis, linear regression models were employed to evaluate associations between DOAC concentrations and several clinical characteristics (previous VKA dose, CrCl, body mass index [BMI], age, and sex). The studied characteristics were analyzed both continuously and categorically, where applicable. The following categories were used for previous daily VKA dose: low doses were classified as ≤1.5 mg for phenprocoumon and ≤2.0 mg for acenocoumarol, medium doses were classified as 1.5–3.0 mg for phenprocoumon and 2.0–3.0 mg for acenocoumarol, and high doses were classified as ≥3.0 mg for phenprocoumon and ≥3.0 mg for acenocoumarol. For impaired kidney function, creatinine clearance of both ≤60 ml/min and ≤50 ml/min were used as cut‐offs. BMI was categorized according to the World Health Organization definitions (<18.5 is underweight, 18.5–24.9 is normal weight, 25–29.9 is overweight, >30 is obese). 23 , 24 Correlations between rivaroxaban/apixaban concentrations and PT and between APTT and dabigatran concentrations were estimated by calculating coefficients of determination (R 2) and linear regression equitation of the fitted regression lines in scatter plots. Adjustments were made for the use of reduced‐dose DOAC when kidney function was studied. All statistical analyses were performed with SPSS® Statistics (IBM SPSS Statistics for Windows, version 25.0), R program (R Foundation for Statistical Computing; available online at https://www.R‐project.org/), and GraphPad Prism (version 8.4.2; GraphPad Software Inc.).
3. RESULTS
3.1. Baseline characteristics
In total, 529 patients were asked for study participation between March 2018 and March 2020, of whom 157 patients (30%, and mainly Caucasian) agreed to participate. Before analyzing the data, we excluded 3 patients due to uncertainty about the timing of DOAC intake, and 2 patients due to the availability of only one DOAC measurement.
Hence, for analysis, we included 152 patients, of whom 51 used rivaroxaban, 50 apixaban, and 51 dabigatran. The average age in the study population was 73.9 ± 8.4 years and 63.2% (96/152) were men (Table 1). Patients were treated with DOAC for atrial fibrillation (83.6%; 127 /152), venous thromboembolism (17.8%; 27/152), and/or other indications (10.5%; 16/152). For the total study population, the average BMI was 27.2 ± 4.7 kg/m² and the average creatinine clearance was 94.4 ± 133.5 ml/min. Before switching to a DOAC, 35 patients (23%) had used acenocoumarol, of whom the majority (37.1%; n = 13/35), used a dosage of 2–3 mg once per day; 117 (77%) patients had used phenprocoumon, of whom the majority (51.3%; n = 60/117) used a dosage of 1.5–3.0 mg once per day. Of the 51 rivaroxaban users, 42 patients (82%) used 20 mg once daily, 6 patients (12%) used 15 mg once daily, and 3 patients (6%) used 10 mg once daily. Of the 50 apixaban users, 45 patients (90%) used 5 mg twice daily, and 5 patients (10%) used 2.5 mg twice daily. Of the 51 dabigatran users, 32 patients (63%) used 150 mg twice daily, and 19 patients (37%) used 110 mg twice daily. Information about the baseline characteristics stratified per DOAC type is presented in Table 1.
TABLE 1.
Baseline characteristics
| All patients a | Rivaroxaban | Apixaban | Dabigatran | |||||
|---|---|---|---|---|---|---|---|---|
| Total | 152 | (100) | 51 | (34) | 50 | (33) | 51 | (33) |
| Sex | ||||||||
| Men, n (%) | 96 | (63) | 38 | (75) | 26 | (52) | 32 | (63) |
| Women, n (%) | 56 | (37) | 13 | (26) | 24 | (48) | 19 | (37) |
| Age, year mean (SD) | 73.9 | (8.4) | 73.8 | (7.3) | 74.6 | (9.6) | 73.3 | (8.4) |
| <45 years, n (%) | 1 | (1) | 0 | (0) | 1 | (2) | 0 | (0) |
| 45–54 years, n (%) | 3 | (2) | 0 | (0) | 1 | (2) | 2 | (4) |
| 55–64 years, n (%) | 9 | (6) | 5 | (10) | 1 | (2) | 3 | (6) |
| 65–74 years, n (%) | 65 | (43) | 24 | (47) | 19 | (38) | 22 | (43) |
| 75–84 years, n (%) | 60 | (40) | 18 | (35) | 21 | (42) | 21 | (41) |
| ≥85 years, n (%) | 14 | (9) | 4 | (8) | 7 | (14) | 3 | (6) |
| Body mass index, kg/m2 mean (SD) | 27.2 | (4.7) | 27.3 | (5.5) | 26.9 | (4.1) | 27.5 | (4.5) |
| <18.5 kg/m2 | 1 | (1) | 0 | (0) | 1 | (2) | 0 | (0) |
| 18.5–24.9 kg/m2 | 51 | (34) | 20 | (39) | 19 | (38) | 12 | (24) |
| 25–29.9 kg/m2 | 72 | (48) | 21 | (41) | 20 | (40) | 31 | (62) |
| >30 kg/m2 | 27 | (18) | 10 | (20) | 10 | (20) | 7 | (14) |
| DOAC dose | ||||||||
| Rivaroxaban 20 mg od | 42 | (28) | 42 | (82) | NA | NA | NA | NA |
| Rivaroxaban 15 mg od | 6 | (4) | 6 | (12) | NA | NA | NA | NA |
| Rivaroxaban 10 mg od | 3 | (2) | 3 | (6) | NA | NA | NA | NA |
| Apixaban 5 mg bid | 45 | (30) | NA | NA | 45 | (90) | NA | NA |
| Apixaban 2.5 mg bid | 5 | (3) | NA | NA | 5 | (10) | NA | NA |
| Dabigatran 150 mg bid | 32 | (21) | NA | NA | NA | NA | 32 | (63) |
| Dabigatran 110 mg bid | 19 | (13) | NA | NA | NA | NA | 19 | (37) |
| Anticoagulation indication c | ||||||||
| Atrial fibrillation, n (%) | 127 | (84) | 39 | (77) | 44 | (88) | 44 | (86) |
| Venous thromboembolism, n (%) | 27 | (18) | 12 | (24) | 7 | (14) | 8 | (16) |
| Other, n (%) | 16 | (11) | 7 | (14) | 3 | (6) | 6 | (12) |
| Kidney function (creatinine clearance, ml/min), mean (SD) | 94.4 | (133.5) | 98.4 | (161.8) | 103.7 | (203.0) | 93.1 | (106.5) |
| ≤60 ml/min | 36 | (24) | 9 | (18) | 15 | (30) | 12 | (25) |
| >60 ml/min | 114 | (76) | 42 | (82) | 35 | (70) | 37 | (76) |
| ≤50 ml/min | 19 | (13) | 6 | (12) | 7 | (14) | 6 | (12) |
| >50 ml/min | 131 | (87) | 45 | (88) | 43 | 86) | 43 | (88) |
| Previous VKA type and average daily dose b | ||||||||
| acenocoumarol 0–1 mg, n (%) | 1 | (3) | 0 | (0) | 0 | (0) | 1 | (8) |
| acenocoumarol 1–2 mg, n (%) | 11 | (31) | 4 | (29) | 2 | (25) | 5 | (39) |
| acenocoumarol 2–3 mg, n (%) | 13 | (37) | 6 | (43) | 3 | (38) | 4 | (31) |
| acenocoumarol >3 mg, n (%) | 10 | (29) | 4 | (29) | 3 | (38) | 3 | (23) |
| phenprocoumon 0–1.5 mg, n (%) | 48 | (41) | 14 | (38) | 20 | (48) | 14 | (37) |
| phenprocoumon 1.5–3.0 mg, n (%) | 60 | (51) | 20 | (54) | 21 | (50) | 19 | (50) |
| phenprocoumon >3.0 mg, n (%) | 9 | (8) | 3 | (8) | 1 | (2) | 5 | (13) |
Abbreviations: DOAC, direct oral anticoagulant; NA, not applicable; SD, standard deviation; VKA, vitamin K antagonist.
With at least 2 available measurements.
1 tablet acenocoumarol equals 1 mg; 1 tablet phenprocoumon equals 3 mg.
In case of multiple indications present, all indications were counted separately.
3.2. Sample availability
The numbers of available blood samples per study contact moment are presented in Table 2. Reasons for unavailability of one or more blood samples were: (1) unwillingness to have further blood withdrawals or failed venipuncture (n = 7); (2) switching back to VKA, switching to platelet inhibitors, or stopping all anticoagulants during the follow‐up (n = 5); (3) unable to go on second or third house visit due to national lockdown measures during the COVID‐19 pandemic in March 2020 (n = 19); (4) other reasons (n = 3). For the two patients who switched from rivaroxaban to apixaban during the follow‐up, only the samples from the initial DOAC were used for analysis.
TABLE 2.
Plasma levels (ng/ml) for DOACs at different time points
| DOAC concentration (ng/ml) | Mean (min‐max) | CV (%) | ||||
|---|---|---|---|---|---|---|
| Number of samples | Mean (min‐max) | CV (%) | Number of samples | |||
| Baseline trough | Baseline peak | |||||
| Rivaroxaban 20 mg od | 42 | 43.83 (3–175) | 79% | 42 | 298.20 (139–434) | 25% |
| Rivaroxaban 15 mg od | 6 | 50.76 (6–221) | 165% | 5 | 253.24 (184–366) | 31% |
| Rivaroxaban 10 mg od | 3 | 14.25 (10–17) | 25% | 3 | 171.62 (123–219) | 28% |
| Apixaban 5 mg bid | 45 | 99.92 (20–281) | 58% | 44 | 209.30 (71–484) | 41% |
| Apixaban 2.5 mg bid | 5 | 69.38 (27–169) | 84% | 5 | 141.29 (86–266) | 51% |
| Dabigatran 150 mg bid | 32 | 89.39 (28–293) | 55% | 32 | 214.09 (58–497) | 60% |
| Dabigatran 110 mg bid | 19 | 87.79 (34–213) | 60% | 19 | 143.42 (43–286) | 46% |
| 2 weeks trough | 2 weeks peak | |||||
| Rivaroxaban 20 mg od | 39 | 40.48 (2–163) | 76% | 38 | 264.77 (28–460) | 31% |
| Rivaroxaban 15 mg od | 5 | 37.52 (17–109) | 107% | 5 | 235.90 (161–330) | 27% |
| Rivaroxaban 10 mg od | 3 | 31.73 (3–56) | 84% | 2 | 171.29 (153–190) | 15% |
| Apixaban 5 mg bid | 43 | 95.80 (30–219) | 49% | 45 | 206.28 (83–385) | 36% |
| Apixaban 2.5 mg bid | 5 | 67.13 (31–137) | 60% | 5 | 111.79 (50–236) | 65% |
| Dabigatran 150 mg bid | 29 | 97.82 (35–324) | 69% | 29 | 203.74 (48–519) | 61% |
| Dabigatran 110 mg bid | 16 | 101.38 (30–352) | 81% | 16 | 154.98 (54–580) | 84% |
| 2 months trough | 2 months peak | |||||
| Rivaroxaban 20 mg od | 34 | 46.06 (13–201) | 81% | 34 | 291.28 (151–437) | 27% |
| Rivaroxaban 15 mg od | 5 | 26.52 (9–42) | 51% | 4 | 232.78 (150–370) | 42% |
| Rivaroxaban 10 mg od | 3 | 6.54 (3–13) | 86% | 3 | 157.59 (111–241) | 46% |
| Apixaban 5 mg bid | 42 | 94.70 (19–209) | 48% | 42 | 201.51 (73–438) | 37% |
| Apixaban 2.5 mg bid | 5 | 59.17 (18–116) | 62% | 5 | 121.52 (67–196) | 48% |
| Dabigatran 150 mg bid | 24 | 99.83 (41–344) | 68% | 24 | 208.02 (60–591) | 69% |
| Dabigatran 110 mg bid | 11 | 99.63 (31–245) | 83% | 11 | 143.41 (53–453) | 80% |
Stratified by DOAC dose and type (inter‐individual variability).
Abbreviations: bid, twice a day; CV, coefficient of variation; DOAC, direct oral anticoagulant; max, maximum; min, minimum; od, once a day.
3.3. Inter‐individual variability
The inter‐individual variability expressed as CV is presented in Table 2 for each DOAC, stratified by DOAC dose. For rivaroxaban 20 mg, CV values ranged from 76% to 81% at trough concentrations and from 25% to 31% for peak concentrations. For apixaban 5 mg, CV values ranged from 48% to 58% for trough concentrations and from 36% to 41% for peak concentrations. For dabigatran 150 mg, CV values ranged from 55% to 69% for trough concentrations and from 60% to 69% for peak concentrations. The CVs for all reduced‐dose DOACs are also presented in Table 2. All available DOAC trough and peak concentrations for the full‐dose DOACs are presented in Figure 1, together with their 20th–80th percentiles.
FIGURE 1.
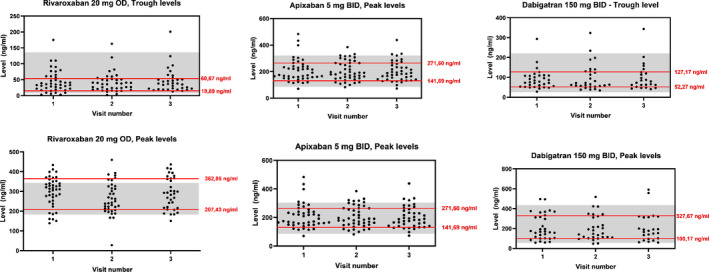
All available direct oral anticoagulant (DOAC) trough and peak levels for the full‐dose DOACs. The red lines represent the 20th and 80th percentiles. The 20th and 80th percentiles are also used in previous studies. 9 The gray areas represent the “on‐therapy” ranges. 19 , 25 BID, twice a day; OD, once a day
3.4. Intra‐individual variability
Intra‐individual variability (CV) was calculated at trough and peak for each patient separately for all available DOAC measurements (with a minimum of two available measurements required) during follow‐up (Table 3). For rivaroxaban, the average CV was 33% (trough) and 17% (peak) for the 20 mg dosage, 37% (trough) and 22% (peak) for the 15 mg dosage, and 92% (trough) and 19% (peak) for the 10 mg dosage. For apixaban, the average CV was 18% (trough) and 15% (peak) for the 5 mg dosage, and 21% (trough) and 20% (peak) for the 2.5 mg dosage. For dabigatran, the average CV was 18% (trough) and 29% (peak) for the 150 mg dosage, and 23% (trough) and 26% (peak) for the 110 mg dosage (Table 3). To visualize the intra‐individual variability, we showed the course of the DOAC concentrations of all patients who had one or more measurements outside the 20th–80th percentile (Figure 2). In general, it often was the same patients who had multiple values either above or below the 20th–80th percentile range. The proportions of patients outside the 20th–80th range were higher for the peak concentrations (82%, 70%, 65%) than for the trough concentrations (71%, 56%, 57%) for rivaroxaban, apixaban, and dabigatran, respectively (Table 4). In contrast, the proportions of patients outside the 20th–80th range ≥2 times were higher for the trough concentrations (50%, 64%, 55%) than for the peak concentrations (33%, 51%, 46%) for rivaroxaban, apixaban, and dabigatran, respectively.
TABLE 3.
Intra‐individual DOAC variability, stratified by DOAC dose and type
| Trough | Peak | |||
|---|---|---|---|---|
| Number of samples | CV (%) | Number of samples | CV (%) | |
| Rivaroxaban 20 mg od | 40 | 33% | 40 | 17% |
| Rivaroxaban 15 mg od | 5 | 37% | 5 | 22% |
| Rivaroxaban 10 mg od | 3 | 92% | 3 | 19% |
| Apixaban 5 mg bid | 43 | 18% | 45 | 15% |
| Apixaban 2.5 mg bid | 5 | 21% | 5 | 20% |
| Dabigatran 150 mg bid | 30 | 18% | 30 | 29% |
| Dabigatran 110 mg bid | 16 | 23% | 16 | 26% |
Abbreviations: bid, twice a day; CV, coefficient of variation; DOAC, direct oral anticoagulant; od, once a day.
FIGURE 2.

Direct oral anticoagulant (DOAC) activity levels of all patients who had one or more measurements outside the 20th–80th percentile. The gray areas represent the values between the 20th and 80th percentiles (of the full‐dose DOACs) . The red lines represent patients with more than one outlier outside the 20th–80th range (of the full‐dose DOACs), the black lines represent patients with one outside the 20th–80th range (of the full‐dose DOACs). BID, twice a day; OD, once a day
TABLE 4.
Percentage of patients with DOAC concentrations outside range a , stratified by DOAC type and moment of measurement
| DOAC type and measurement | Number of patients outside range b (%) | Outside range ≥2 times (%) |
|---|---|---|
| Rivaroxaban, trough levels | 36/51 (71%) | 18/36 (50%) |
| Rivaroxaban, peak levels | 42/51 (82%) | 14/42 (33%) |
| Apixaban, trough levels | 28/50 (56%) | 18/28 (64%) |
| Apixaban, peak levels | 35/50 (70%) | 18/35 (51%) |
| Dabigatran, trough levels | 29/51 (57%) | 16/29 (55%) |
| Dabigatran, peak levels | 33/51 (65%) | 15/33 (46%) |
Abbreviation: DOAC, direct oral anticoagulant.
Within range is defined as a value within the 20th and 80th percentile (of full dose DOAC).
At least once.
3.5. Correlation of DOAC plasma trough concentrations and clinical characteristics
There was an inverse correlation present between the previous mean daily VKA dose and DOAC trough concentrations for all DOACs, which was statistically significant for rivaroxaban. For rivaroxaban, the beta (β) was −20.9 (95% confidence interval [95% CI] −34.2 to −7.7) and the R‐value was −0.41 (Table 5). For apixaban, the β was −14.7 (95% CI −35.5 to 6.0) and the R‐value was −0.20. For dabigatran, the β was −10.6 (95% CI −32.6 to 11.4) and the R‐value was −0.14 (Table 5). The median, quartiles, and ranges from all DOAC concentrations, stratified by previous VKA group, are presented for each DOAC in Figure 3. There also was an inverse correlation present between creatinine clearance and DOAC trough concentrations for all DOACs, though again only statistically significant for rivaroxaban. For rivaroxaban, the β was −33.1 (95% CI −56.7 to −9.4) and the R‐value was −0.37. For apixaban, the β was −12.2 (95% CI −40.8 to 16.5) and the R‐value was −0.12. For dabigatran, the β was −18.0 (95% CI −55.3 to 19.1) and the R‐value was −0.14. Results were similar when creatinine clearance was used in the regression model as a continuous outcome. The inverse correlation between DOAC concentration and creatinine clearance became more evident for all DOAC types after adjustments for reduced‐dose DOACs.
TABLE 5.
Subgroup trough levels, linear regression analysis
| Rivaroxaban | Apixaban | Dabigatran | ||||
|---|---|---|---|---|---|---|
| R | β (95% CI) | R | β (95% CI) | R | β (95% CI) | |
| Previous VKA dose a | ||||||
| Crude model, categorical | −0.41 | −20.9 (−34.2 to −7.7) | −0.20 | −14.7 (−35.5 to 6.0) | −0.14 | −10.6 (−32.6 to 11.4) |
| Kidney function | ||||||
| Crude model, continuous | −0.28 | −0.2 (−0.5 to −0.001) | −0.19 | −0.04 (−0.1 to 0.02) | −0.10 | −0.05 (−0.2 to 0.1) |
| Crude model, categorical 1 | −0.37 | −33.1 (−56.7 to −9.4) | −0.12 | −12.2 (−40.8 to 16.5) | −0.14 | −18.0 (−55.3 to 19.3) |
| Crude model, categorical 2 | −0.19 | −20.0 (−49.6 to 9.6) | −0.23 | −29.9 (−67.1 to 7.2) | −0.21 | −34.8 (−83.1 to 13.5) |
| Adjusted model c , continuous | −0.30 | −0.2 (−0.5 to −0.01) | −0.24 | −0.03 (−0.1 to 0.04) | −0.10 | −0.05 (−0.2 to 0.1) |
| Adjusted model c , categorical 1 | −0.43 | −39.6 (−64.2 to −15.1) | −0.30 | −22.7 (−52.7 to 7.2) | −0.16 | −22.3 (−64.4 to 19.9) |
| Adjusted model c , categorical 2 | −0.24 | −25.1 (−56.2 to 6.0) | −0.41 | −52.3 (−91.5 to −13.2) | −0.24 | −45.3 (−101.0 to 10.4) |
| BMI | ||||||
| Crude model, continuous | −0.04 | −0.3 (−2.0 to 1.5) | −0.04 | −0.4 (−3.7 to 2.9) | 0.41 | 5.1 (1.8 to 8.4) |
| Crude model, categorical | 0.04 | 2.0 (−11.1 to 15.1) | −0.01 | −0.4 (−17.3 to 16.5) | 0.33 | 30.1 (5.4 to 54.8) |
| Age | ||||||
| Crude model, continuous | 0.10 | 0.5 (−0.9 to 1.8) | 0.26 | 1.3 (−0.1 to 2.6) | 0.05 | 0.4 (−1.5 to 2.2) |
| Crude model, categorical | 0.10 | 6.6 (−12.9 to 26.2) | 0.24 | 22.2 (−3.6 to 47.9) | −0.18 | −20.2 (−51.6 to 11.2) |
| Sex b | ||||||
| Crude model, categorical | −0.08 | −6.2 (−28.4 to 16.0) | 0.06 | 5.2 (−21.3 to 31.6) | 0.24 | 27.2 (−4.1 to 58.5) |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; R, correlation coefficient; VKA, vitamin K antagonist; β, Beta.
For previous VKA dose, the continuous analysis was not possible due to differences number of tablets of in previous VKA type (acenocoumarol or phenprocoumon).
The reference group is male sex.
Adjusted for low‐dose DOAC.
Using a cut‐off for creatinine clearance of 60 ml/min.
Using a cut‐off for creatinine clearance of 50 ml/min.
FIGURE 3.
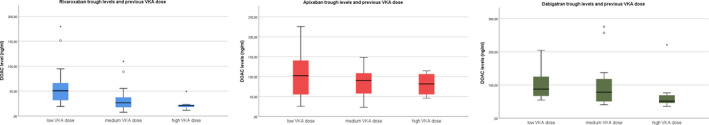
Correlations between direct oral anticoagulant (DOAC) plasma trough levels and previous vitamin K antagonist (VKA) dose
The median, quartiles, and ranges from all DOAC concentrations, stratified by creatinine clearance group, are presented for each DOAC in Figure 4. For the relationship between BMI and DOAC trough concentrations, there was no clear correlation identified, except for dabigatran. For rivaroxaban, the β was 2.0 (95% CI −11.1 to 15.1) and the R‐value was 0.04. For apixaban, the β was −0.4 (95% CI −17.3 to 16.5) and the R‐value was −0.01. For dabigatran, the β was 30.1 (95% CI −5.4 to 54.8) and the R‐value was 0.33 (Table 5). The results were similar when BMI was used in the regression model as a continuous outcome. The median, quartiles, and ranges from all DOAC concentrations, stratified by BMI group, are presented for each DOAC in Figure 5. The results for the variables age and sex are presented in Table 5. Results remained similar for all studied outcomes after adjustments for anticoagulation indication (Table S2 in supporting information). Similar results were found when correlations between DOAC peak concentrations and all clinical characteristics were studied (Table S1 in supporting information), although the correlations were less strong.
FIGURE 4.

Correlations between direct oral anticoagulant (DOAC) plasma trough levels and creatinine clearance
FIGURE 5.

Correlations between direct oral anticoagulant (DOAC) plasma trough levels and body mass index (BMI)
3.6. Correlation between DOAC plasma concentration and PT or APTT
Scatter plots showing DOAC concentrations and PT or APTT for all six moments of blood withdrawal are shown in Figures [Link], [Link], [Link] in supporting information. Overall, correlations between DOAC concentrations and PT or APTT were moderate to good. The R 2 between rivaroxaban and PT ranged between 0.39 and 0.82, R 2 between apixaban and PT ranged between 0.39 and 0.75, and R 2 between dabigatran and APTT ranged between 0.43 and 0.69.
4. DISCUSSION
In this study, the inter‐ and intra‐individual variability of DOAC concentrations in patients on VKA who were switched to DOAC was studied. We found substantial inter‐individual variability, which was more evidently present than intra‐individual variability. Furthermore, we identified some clinical characteristics that can predict high or low DOAC concentrations (creatinine clearance and previous VKA dosage).
Inter‐individual DOAC drug concentration variability has been studied previously and authors have reported both low and high DOAC variability. 8 , 9 , 10 For example, in studies with data from the clinical DOAC trials, edoxaban 13 and dabigatran 12 concentrations showed variation between individuals. In our study, substantial inter‐individual variability was present for all studied DOACs, with CVs ranging from 48% to 81% for trough concentrations and from 25% to 69% for peak concentrations with full‐dose DOAC. For rivaroxaban and apixaban, trough concentrations showed higher variability than peak concentrations. These results are consistent with a comparable pharmacokinetic study that showed similar inter‐individual variability in rivaroxaban, apixaban, and dabigatran patients in daily practice. 10 Results of that study showed similar CVs and reported the highest variability for dabigatran. They also found considerably higher variability in patients with reduced‐dose DOAC, which finding we confirmed in our study: in reduced‐dose DOAC patients higher variability was found (maximum of 165% for trough concentrations, and maximum of 84% for peak concentrations), but numbers were small and therefore this result should be handled with caution.
In our study, the intra‐individual variability ranged between 18% and 33% for trough concentrations, and between 15% and 29% for peak concentrations for the standard‐dose DOACs, which is substantially lower than the inter‐individual variability. These findings are consistent with the study by Gulpen et al., who reported marked DOAC concentration variation seen between patients and no significant variation within patients over time among 164 rivaroxaban or dabigatran patients. 9
As patients’ daily DOAC dose did not change during the study (patients received either standard or reduced‐dose DOAC), it is remarkable that such substantial variability was identified. For anticoagulants, where insufficient dosage means reduced benefit in thromboembolic prevention and overdosage increases the risk of bleeding, high variability is not a desirable characteristic. A therapeutic window, or “target range” in which the optimal balance between clotting and bleeding (lowest risk of both outcomes) is achieved, is currently not known for DOAC. In the absence of such a therapeutic range, previous and current studies about DOAC concentrations usually report DOAC values relative to the 5th–95th percentile ranges obtained from phase II‐III trials, which are hence used as a surrogate for the “therapeutic range.” 19 , 25 An important difference with a therapeutic range is that these ranges represent DOAC concentrations of the majority of patients in those studies, but do not show the relationship between DOAC concentrations and clinical outcomes, which are the most relevant. Available evidence, however, suggests the existence of such a “target range” for DOACs, suggesting that low DOAC concentrations increase the risk of thrombosis, and high DOAC concentrations increase the risk of bleeding. 12 , 13 For example, the Randomized Evaluation of Long‐Term Anticoagulant Therapy (RE‐LY) trial compared the efficacy and safety of dabigatran with warfarin among atrial fibrillation patients, and identified an inverse correlation between the risk of ischemic events and dabigatran trough concentrations (c‐statistic 0.66, 95% CI 0.61–0.71), and between the risk of major bleeding and dabigatran trough concentrations (c‐statistic 0.72, 95% CI 0.69–0.74).
The high variability we identified between individuals suggests that some patients may be exposed to DOAC drug concentrations that are too high or too low for them as an individual and therefore they may have a higher risk of either bleeding or thrombosis. To reduce such complications, the results of our study suggest two approaches: first, it may be worthwhile to explore if measuring DOAC concentrations at initiation of treatment in daily clinical practice is beneficial. If a patient’s DOAC concentrations are then considered optimal, this patient may not need frequent remeasuring as DOAC concentrations within an individual appear to be more stable (for example only once per year, or during sudden changes in interacting medication or comorbidity). This remeasuring may be done at the same time that the kidney function of a DOAC patient is monitored, which should be done at least once per year according to clinical guidelines. Whether DOAC trough or peak concentrations and which laboratory assay (Anti‐Xa assays, dTT, or PT/APTT) could be used best remains to be further studied, as this depends on the correlation with clinical outcomes.
As a second approach, identifying clinical characteristics that predict high or low DOAC concentrations could be valuable as a basis for an individual DOAC dosing regimen. We therefore studied three potential predictors for high or low DOAC trough concentrations: previous VKA dose, creatinine clearance, and BMI. Of note, our study was not powered to look at these differences and results should be handled with caution as they are based on post hoc analyses. First, we found an inverse correlation between previous average daily VKA dose and DOAC trough concentration. For VKA, many factors are known to influence the international normalized ratio (INR). 26 A (partial) overlap between factors that could influence INR and DOAC trough concentrations, such as a genetic variation in metabolizing medication, may explain our finding. This therefore may be interesting for further exploration in follow‐up studies. To our knowledge, there are no other studies available that have investigated previous average VKA dose as a predictor for DOAC concentrations. Second, we showed an inverse correlation between creatinine clearance and DOAC trough concentrations. All available DOACs have some level of renal excretion. Therefore current guidelines exclude their use in patients with a creatinine clearance <30 ml/min for dabigatran, advise dose adjustments with a creatinine clearance <50 ml/min for dabigatran and rivaroxaban, and advise dose adjustments in patients with a creatinine clearance <30 ml/min for apixaban. 27 In our study, three patients were treated with full‐dose rivaroxaban, while their creatinine clearance was <50 ml/min. The trough levels (163–201 ng/ml) of the patient with the lowest creatinine clearance (47 ml/min) widely exceeded the 20th–80th percentile range (19–70 ng/ml), which is likely to have been caused by the inappropriate DOAC dose. The renal excretion likely explains the inverse correlation and provides an interesting opportunity for future studies. Patients with moderately impaired kidney function who do not directly have a contraindication for DOAC treatment may be still more likely to have higher DOAC trough concentrations as suggested by our study and therefore be at increased risk of bleeding. 12 It could be worthwhile to study whether, for example at therapy initiation or after a sudden change in kidney function, a check of DOAC concentration and possibly adjustment of dosage would be beneficial in terms of preventing thrombosis or bleeding. In addition, renal clearance may be useful as an independent variable in risk prediction models for bleeding or thrombosis while using DOACs. Of note, in other studies, the associations between kidney function and rivaroxaban or apixaban concentrations could not be identified, 10 which warrants a careful approach of our study results that relate to this issue as opposed to other studies. A potential explanation might be a lack of statistical power in our analyses—as in the analyses of previous studies that looked at the correlation between kidney levels and DOAC concentrations. Third, we could not identify a clear correlation between BMI levels and DOAC trough concentrations, which is similar to another study that relates to this issue, 28 but differs from a study that demonstrated a negative association between DOAC concentration and BMI for dabigatran and apixaban. 29 Whether DOACs can be safely used in patients with obesity is currently not well known, because patients with the highest weight were not well represented in clinical trials. Still, several observational studies have demonstrated their safety and efficacy in this population. 30 , 31 As the number of patients with morbid obesity (BMI > 35) in our study was limited, these results should be interpreted with caution.
Our study has several strengths. First, our structured follow‐up of participants allowed not only a comparison of DOAC concentrations between but also within patients for the three most commonly used DOACs in the Netherlands. Also, due to listing the timing of DOAC intake, we were able to precisely calculate the time between last intake and DOAC trough or peak concentrations, which allowed a more accurate comparison of DOAC concentrations to studies in which the exact time of last DOAC intake was not well known. Third, to lower the influence of day‐to‐day variation all measurements of the same DOAC were performed on the same day. In addition, all unexpected results were remeasured one or two times to ensure that the values were consistent.
Some limitations of the study need to be acknowledged. First, our study population consisted only of patients who previously used VKAs, and results may therefore not be generalizable to anticoagulation‐naïve patients. Second, peak concentrations were measured 2 h after DOAC administration. For dabigatran and rivaroxaban, peak concentrations could also be reached after 2–4 h 32 , 33 and for apixaban after 3–4 h. 34 As a result, some patients may have had a higher peak concentration than was measured in our study. Also, due to the smaller number of patients in the subgroups and the nature of a post hoc analysis, the results of the three explanatory analyses must be interpreted with caution. Due to the national lockdown measures due to the COVID‐19 pandemic from March 2020 onward, for several dabigatran and rivaroxaban patients, the second or third visit could not take place, and could not be rescheduled. These missing visits are, however, most likely at random and enough people were included who had all three visits (>36 patients) to perform the main analyses.
5. CONCLUSIONS
Substantial inter‐individual variability in DOAC concentrations was identified among former VKA patients who recently switched to DOACs, which was higher than the intra‐individual variability. Lower previous VKA dosage and creatinine clearance were associated with higher DOAC trough concentrations. These findings support further study into an optimal target range and into individualized dosing, to minimize both bleeding and thrombosis in DOAC users.
CONFLICTS OF INTEREST
MMA Toorop, N van Rein, FJM van der Meer, MC Nierman, HW Vermaas, SC Cannegieter, and WM Lijfering have nothing to disclose. MV Huisman reports grants from ZonMW Dutch Healthcare Fund, and grants and personal fees from Boehringer‐Ingelheim, Pfizer‐BMS, Bayer Health Care, Aspen, Daiichi‐Sankyo, outside the submitted work.
AUTHOR CONTRIBUTIONS
MMA Toorop and WM Lijfering designed the research. MMA Toorop, FJM van der Meer, MC Nierman, and HW Vermaas collected the data. MMA Toorop analyzed the data. MMA Toorop wrote the manuscript. N van Rein, SC Cannegieter, WM Lijfering, FJM van der Meer, MC Nierman, and HW Vermaas revised the paper for important intellectual content.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1‐S2
Toorop MMA, van Rein N, Nierman MC, et al. Inter‐ and intra‐individual concentrations of direct oral anticoagulants: The KIDOAC study. J Thromb Haemost. 2021;20:92–103. doi: 10.1111/jth.15563
Manuscript handled by: Robert Gosselin
Final decision: Robert Gosselin, 15 October 2021
REFERENCES
- 1. Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van den Heuvel JM, Hovels AM, Buller HR, Mantel‐Teeuwisse AK, de Boer A, Maitland‐van der Zee AH. NOACs replace VKA as preferred oral anticoagulant among new patients: a drug utilization study in 560 pharmacies in The Netherlands. Thromb J. 2018;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connoly SJ, Ezekowitz M, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139‐1151. [DOI] [PubMed] [Google Scholar]
- 4. Patel M, Mahaffey K, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrilation. N Engl J Med. 2011;365:883‐891. [DOI] [PubMed] [Google Scholar]
- 5. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981‐992. [DOI] [PubMed] [Google Scholar]
- 6. Graham DJ, Baro E, Zhang R, et al. Comparative stroke, bleeding, and mortality risks in older medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(596–604):e11. [DOI] [PubMed] [Google Scholar]
- 7. FNT . De kunst van het doseren. Accessed May 1, 2021. https://wwwfntnl/kwaliteit/de‐kunst‐van‐het‐doseren
- 8. Chan NC, Coppens M, Hirsh J, et al. Real‐world variability in dabigatran levels in patients with atrial fibrillation. J Thromb Haemost. 2015;13:353‐359. [DOI] [PubMed] [Google Scholar]
- 9. Gulpen AJW, Ten Cate H, Henskens YMC, et al. The daily practice of direct oral anticoagulant use in patients with atrial fibrillation; an observational cohort study. PLoS One. 2019;14:e0217302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Testa S, Tripodi A, Legnani C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res. 2016;137:178‐183. [DOI] [PubMed] [Google Scholar]
- 11. Toorop MMA, Lijfering WM, Scheres LJJ. The relationship between DOAC levels and clinical outcomes: the measures tell the tale. J Thromb Haemost. 2020;18:3163‐3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY Trial (Randomized Evaluation of Long‐Term Anticoagulation Therapy). J Am Coll Cardiol. 2014;63:321‐328. [DOI] [PubMed] [Google Scholar]
- 13. Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti‐Factor Xa activity, and outcomes: an analysis of data from the randomised, double‐blind ENGAGE AF‐TIMI 48 trial. Lancet. 2015;385:2288‐2295. [DOI] [PubMed] [Google Scholar]
- 14. Testa S, Legnani C, Antonucci E, et al. Drug levels and bleeding complications in atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2019;17:1064‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Testa S, Paoletti O, Legnani C, et al. Low drug levels and thrombotic complications in high‐risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16:842‐848. [DOI] [PubMed] [Google Scholar]
- 16. Zhao X, Sun P, Zhou Y, et al. Safety, pharmacokinetics and pharmacodynamics of single/multiple doses of the oral, direct Factor Xa inhibitor rivaroxaban in healthy Chinese subjects. Br J Clin Pharmacol. 2009;68:77‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fawzy AM, Lip GYH. Pharmacokinetics and pharmacodynamics of oral anticoagulants used in atrial fibrillation. Expert Opin Drug Metab Toxicol. 2019;15:381‐398. [DOI] [PubMed] [Google Scholar]
- 18. Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2017;16:209‐219. [DOI] [PubMed] [Google Scholar]
- 19. Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118:437‐450. [DOI] [PubMed] [Google Scholar]
- 20. Curvers J, van de Kerkhof D, Stroobants AK, van den Dool EJ, Scharnhorst V. Measuring direct thrombin inhibitors with routine and dedicated coagulation assays: which assay is helpful? Am J Clin Pathol. 2012;138:551‐558. [DOI] [PubMed] [Google Scholar]
- 21. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31‐41. [DOI] [PubMed] [Google Scholar]
- 22. Mueck W, Lensing AWA, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Population pharmacokinetic analyses in patients treated for acute deep‐vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675‐686. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization . Obesity. Accessed May 1, 2021. https://wwwwhoint/health‐topics/obesity#tab=tab_1
- 24. World Health Organization . Prevalence of underweight among adults BMI<18.5. Accessed May 1, 2021. https://www.who.int/data/gho/indicator‐metadata‐registry/imr‐details/4804
- 25. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate–a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 26. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and Management of the Vitamin K Antagonists. Chest. 2008;133:160S‐S198. [DOI] [PubMed] [Google Scholar]
- 27. Parker K, Thachil J. The use of direct oral anticoagulants in chronic kidney disease. Br J Haematol. 2018;183:170‐184. [DOI] [PubMed] [Google Scholar]
- 28. Barsam SJ, Patel JP, Roberts LN, et al. The impact of body weight on rivaroxaban pharmacokinetics. Res Pract Thromb Haemost. 2017;1:180‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borst JM, van Rein N, Bakker ECMD, et al. Body weight is negatively associated with direct oral anticoagulant trough concentrations in dabigatran and apixaban users. Br J Haematol. 2020;191:927‐944. [DOI] [PubMed] [Google Scholar]
- 30. Patil T, Lebrecht M. A single center retrospective cohort study evaluating use of direct oral anticoagulants (DOACs) in morbidly obese veteran population. Thromb Res. 2020;192:124‐130. [DOI] [PubMed] [Google Scholar]
- 31. Kushnir M, Choi Y, Eisenberg R, et al. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single‐centre, retrospective analysis of chart data. Lancet Haematol. 2019;6:e359‐e365. [DOI] [PubMed] [Google Scholar]
- 32. Farmacotherapeutisch Kompas . Rivaroxaban Eigenschappen. Accessed July 1, 2021. https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/r/rivaroxaban
- 33. Farmacotherapeutisch Kompas . Dabigatran Eigenschappen. Accessed July 1, 2021. https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/d/dabigatran
- 34. Farmacotherapeutisch Kompas . Apixaban Eigenschappen. Accessed July 1, 2021. https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/a/apixaban
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1‐S2


