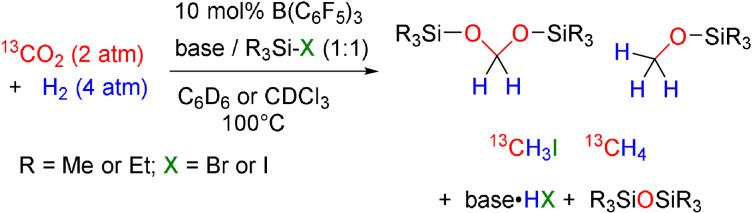
Table 1.
CO2 hydrogenation in the presence of silylhalides.
|
Ent |
Solv. |
Silylhalide[a] |
base[a] |
t [h] |
Major product |
Yield[b] |
|---|---|---|---|---|---|---|
|
1 |
C6D6 |
Me3SiCl |
Lut |
20 |
‐ |
<1 % |
|
2 |
CDCl3 |
Me3SiCl |
Lut |
20 |
‐ |
<1 % |
|
3 |
C6D6 |
Me3SiBr |
Lut |
40 |
MeOSiMe3 |
83 % |
|
4 |
CDCl3 |
Me3SiBr |
Lut |
60 |
MeOSiMe3 |
73 % |
|
5 |
C6D6 |
Me3SiI |
Lut |
60 |
13CH4 |
76 % |
|
6 |
CDCl3 |
Me3SiI |
Lut |
20 |
13CH4 |
85 % |
|
7 |
C6D6 |
Et3SiI |
Lut |
60 |
(Et3SiO)2 13CH2 |
72 % |
|
8 |
CDCl3 |
Et3SiI |
Lut |
40 |
13CH3I |
82 % |
|
9 |
C6D6 |
Et3SiI |
Col |
40 |
(Et3SiO)2 13CH2 |
8 % |
|
10 |
CDCl3 |
Et3SiI |
Col |
40 |
MeOSiEt3 |
9 % |
[a] 0.05 mmol silylhalide and Lewis base were added; Lut=2,6‐lutidine; Col=2,4,6 collidine. [b] Yields are determined by 1H NMR spectroscopy using 10 μL toluene as internal standard.
