Fig. 2 |. Analysis of liganded HER receptor states reveals an allosteric mechanism of dimerization arm engagement.
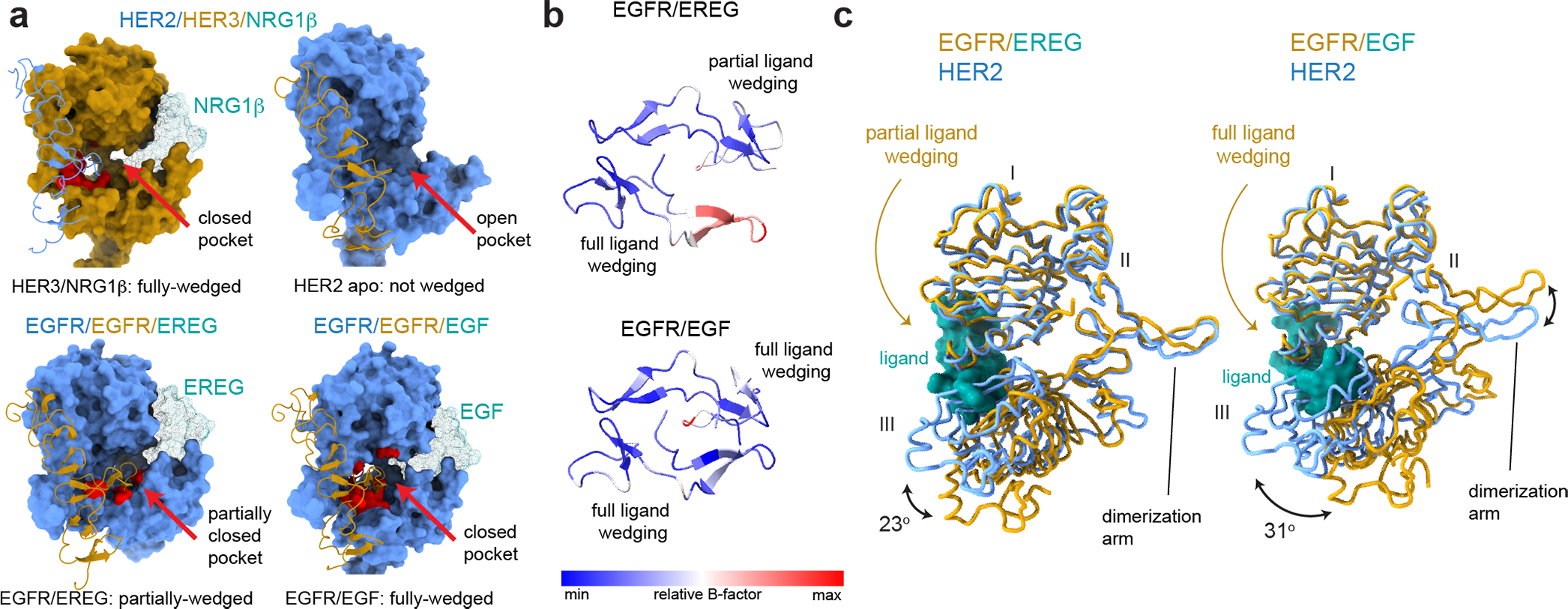
a, Left top panel - closed dimerization arm-binding pocket in HER3 engages the HER2 dimerization arm in the HER2/HER3/NRG1β structure. Right top panel - an open dimerization arm-binding pocket in the ligand-free HER2 does not engage HER3 dimerization arm in the same structure. Left bottom panel – a partially closed dimerization arm-binding pocket in the monomer in the EGFR/EREG structure in which the ligand (EREG) is partially-wedged (PDB ID 5WB7). Right bottom panel – closed binding pocket in EGFR engages a fully-wedged EGF ligand in the EGFR/EGF dimer structure (PDB ID 3NJP). Residues within 4Å of the dimerization arm are shown in red. b, Top view of dimerization arms in the asymmetrically ligand-wedged EGFR/EREG and symmetrically ligand-wedged EGFR/EGF crystal structures indicating different values of B-factors (PDB IDs 5WB7 and 3NJP, respectively). c, Detailed view of domains I-III in the EGFR/EREG or EGFR/EGF crystal structures aligned on HER2 domain I in the HER2/HER3/NRG1β structure. The EGFR monomer in which the EREG ligand is only partially-wedged is shown.
