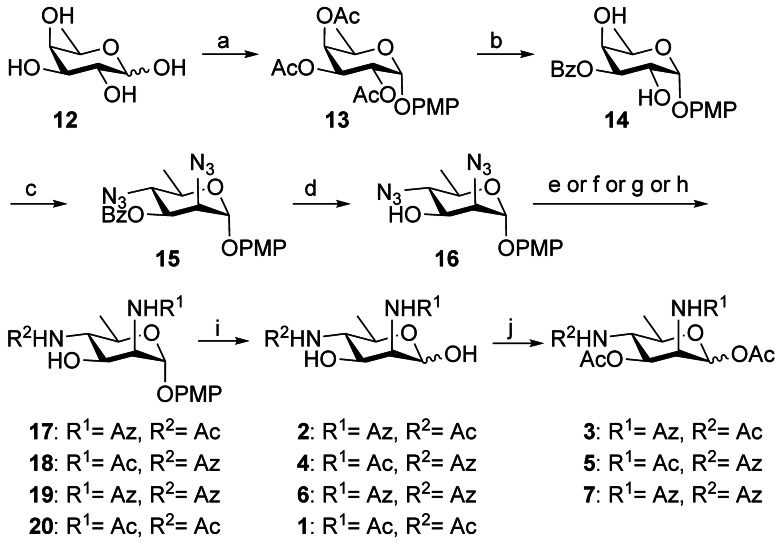
Scheme 2.
Synthesis of Leg precursor 1 and its azido analogues 2–7. Reagents and conditions: a) i) Ac2O, pyridine, 0 °C→rt; ii) PMPOH, BF3⋅Et2O, CH2Cl2, 0 °C→rt, 83 % over two steps; b) i) NaOMe, MeOH, reflux; ii) 2‐aminoethyl diphenylborinate, DIPEA, BzCl, CH3CN, 74 % over two steps; c) i) Tf2O, pyridine, DCM, −10 °C; ii) TBAN3, toluene, 70 °C for 1 h, then 100 °C for 1 h, 84 % over two steps; d) NaOMe, MeOH, reflux, 95 %; e) for 17: i) Pd(OH)2/C, H2, MeOH; ii) EDC, HOBT, NaHCO3, AzOH, CH3CN; iii) Ac2O, MeOH, 0 °C, 40 % over three steps; f) for 18: i) Pd(OH)2/C, H2, MeOH; ii) Ac2O, MeOH, 0 °C; iii) EDC, HOBT, NaHCO3, AzOH, CH3CN; iv) NaOMe, MeOH, 36 % over four steps; g) for 19: i) Pd(OH)2/C, H2, MeOH; ii) EDC, HOBT, NaHCO3, AzOH, CH3CN; iii) NaOMe, MeOH, 55 % over three steps; h) for 20: i) Pd(OH)2/C, H2, MeOH; ii) Ac2O, MeOH, 58 % over two steps; i) CAN, CH3CN/H2O (3:1 v/v), 71 % for 2, 72 % for 4, 60 % for 6, 75 % for 1; j) Ac2O, Et3N, CH2Cl2, 81 % for 3, 85 % for 5, 82 % for 7. Bz=benzoyl, CAN=ceric ammonium nitrate, DCM=dichloromethane, DIPEA=N,N‐diisopropylethylamine, EDC=1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide hydrochloride, HOBT=1‐hydroxy‐1H‐benzotriazole, Tf=trifluoromethanesulfonate.