Abstract
Depression and anxiety often co-occur with cardiac diseases. The Shexiang Baoxin pill (SBP) is a proprietary Chinese medicine initially used to treat cardiac conditions. This study explored whether SBP has antidepressant and anxiolytic effects in addition to hormonal and psychotropic mechanisms. Mice underwent 6 weeks of chronic unpredictable mild stress (CUMS) to induce depression- and anxiety-like behavior. During the 6-week experiment, mice received SBP at intragastric doses of 20.25 mg/kg or 40.5 mg/kg daily. Animals were then tested for depression in sucrose preference, forced-swimming, and tail suspension paradigms, and for anxiety in open field and elevated plus maze tests. Both SBP doses significantly reduced anhedonic behavior in the sucrose preference test; the high SBP dose also increased the number of entries into the central zone of the open field. SBP-treated mice had markedly lower blood levels of corticotrophin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) than stressed mice treated with vehicle. Either low- or high-dose SBP reversed stress-induced reductions of norepinephrine (NE) and dopamine (DA) metabolites and the expression levels of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and glial cell-derived neurotrophic factor (GDNF) in related brain regions. These results suggest that SBP could prevent and alleviate prolonged stress-induced anhedonia and anxiety in association with its suppression of the hypothalamic-pituitary-adrenal (HPA) axis hyperactivity, modulation of brain monoamine neurotransmitter metabolism and neurotrophins. SBP may be particularly suitable for the management of depressive and anxiety disorders in patients with cardiac conditions.
Keywords: Shexiang Baoxin pill (SBP), Depression, Anxiety, HPA axis, BDNF
1. Introduction
Depression and anxiety often co-occur with cardiovascular disease and are common and persistent comorbid symptoms in survivors of acute myocardial infarction [1,2]. Approximately 20% of patients with cardiovascular disease and 40% with coronary heart disease had comorbid depressive disorders [2]. Depression is a risk marker for an increased incidence of new cardiovascular diseases and worse prognosis for existing cardiovascular diseases; a bidirectional association between depression and cardiovascular dysfunction has been well demonstrated [3]. Multisystem mechanisms, such as hypothalamic-pituitary-adrenal (HPA) axis hyperactivity, brain neurotransmitter dysfunction, and neurodegeneration are thought to be involved in the pathogenesis of cardiovascular condition-associated mood and anxiety disorders [4].
The Shexiang Baoxin pill (SBP) is a proprietary Chinese medicine that has been listed in the Chinese Pharmacopoeia since 1995 [5–7]. SBP in composed of seven medicinal materials and was initially developed for the treatment of cardiac conditions [5]. Pharmaceutical quality control for SBP has been well established. Individual materials and major bioactive constituents are listed in Table 1. The clinical efficacy and benefits of SBP have been confirmed in patients with coronary heart disease, atherosclerosis, myocardial ischemia, myocardial fibrosis, angina pectoris, and myocardial infarction, and the therapeutic effects of SBP are suggested to be associated with cytoprotection and immunomodulation [6,7].
Table 1.
Individual Medicinal materials of the SBP formulaa.
| Material name (in Chinese) | Full scientific name | Major pharmacologically active constituents |
|---|---|---|
| Artificial Moschus from musk dear (Ren-Gong-She-Xiang) | The dried preputial secretion of Moschus berezovskii Flerov, Moschus sifanicus Przewalski, or Moschus moschiferus Linnaeus |
Muscone |
| Radix Ginseng (Ren-Shen) | Panax ginseng C.A. Mey., root | Ginsenosides, e.g., ginsenoside Rb1, Rc, Rg1 |
| Cortex Cinnamomi (Rou-Gui) | Cinnamomum cassia (L.) J. Presl., bark | Cinnamic aldehyde, cinnamic acid |
| Venenum Bufonis (toad venom) (Chan-Su) | The dried secretion of Bufo bufo gargarizans Cantor or Bufo melanostictus Schneider |
Bufadienolides, e.g., bufalin, resibufogenin, gamabufotalin |
| Styrax (Su-He-Xiang) | Liquidambar orientalis Mill., resin | Oleanolic acid |
| Artificial Calculus Bovis (Ren-Gong-Niu-Huang) | The dried gall-stone of Bos taurus domesticus Gmelin | |
| Borneol (Bing-Pian) | Borneolum Syntheticum or Dryobalanops aromatica C.F. Gaertn, resin | Borneol |
On the other hand, multiple constituents of SBP have modulatory effects on stress-related neurotransmitters and hormones [8]. Muscone, a principal constituent of musk from the musk dear, and cultivated Calculus bovis protected cerebral neurons and myocardial cells from hypoxia [9]. Cinnamic aldehyde, a major compound derived from Cortex Cinnamomi, exerted its antidepressant effects by inhibiting inflammation in a rat model of chronic unpredictable mild stress (CUMS) [10]. Antidepressant effects of ginsenosides of ginseng have been observed in various animal models [11] and are associated with their broad modulation of stress-related brain monoaminergic neurotransmitters [12]. Oleanolic acid, a key component existing in Styrax spp., and its several derivatives also possess antidepressant and anxiolytic activity by broadly modulating GABAA, DA, and 5-HT receptors and the BDNF signaling pathway [13]. Borneol can promote the blood–brain barrier and epithelial permeability [14,15], and co-administration with borneol elevated blood levels of multiple ginsenosides in SBP [16]. Cotreatment with the artificial Calculus Bovis and haloperidol synergetically enhanced antipsychotic effects [17]. Borneol and muscone behaved as effective absorption enhancers in the Human Nasal Epithelial Cell monolayer by opening the barrier and increasing the paracellular and transcellular transport [18]. Inhalation of SuHeXiang essential oil contained in SBP also could produce the antidepressant and anxiolytic effects [19].
These results have led to the hypothesis that SBP may be also effective in preventing and alleviating stress-induced depression and anxiety. To test this hypothesis, the present study was designed to examine the antidepressant and anxiolytic effects of SBP in a mouse model of CUMS, which has been widely used in the investigation of stress-related disorders [20] and depression-cardiovascular comorbidity [21]. We also examined the hypothalamic-pituitary-adrenal (HPA) axis hormones, brain monoamine neurotransmitters, and multiple neurotrophic factors.
2. Materials and methods
2.1. Animals
All experimental procedures were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (CULATR 3812–15). Female C57BL/6 J mice weighing 18–22 g at 8 weeks of age were purchased from Charles River Laboratory (Wilmington, MA, USA). Mice were housed at a constant temperature (23 ± 2 °C) and maintained on a 12 h/12 h light/dark cycle (lights on 6:00–18:00) with ad libitum access to food and water. All mice were acclimatized for 1 week before the experiment.
2.2. CUMS procedure
The CUMS procedure consisted of multiple different types of mild stressors [22]: tail clamping for 1 min, water deprivation for 15 h, food deprivation for 15 h, restraint in a plastic tube for 4 h, cage tilting at 45° for 15 h, empty cage without nesting for 15 h, illumination in dark phase, and wet bedding (50 g sawdust/200 mL water) for 15 h. The mice received one of these stressors per day, and the same stressor was not applied for 2 consecutive days to minimize the predictability of the occurrence of each stressor. These stressors were randomly assigned on a weekly basis and repeated throughout the 6-weeks experiment. Animals received the SBP treatment during the entire CUMS procedure (see below).
2.3. Drug and treatment regimens
SBP (manufacturer batch number: 160,504) was kindly provided by Shanghai Hutchison Pharmaceuticals Company (Shanghai, China). The quality control of SBP adhered to the specifications and test procedures as described in the Chinese Pharmacopoeia [5]. For administration, SBP was dissolved in a vehicle containing a 0.5% aqueous solution of sodium carboxymethyl cellulose and the two effective doses, 20.25 mg/kg and 40.5 mg/kg per day, were given via oral gavage 30 min before the CUMS stressor on a daily basis. These two effective doses of SBP tested were calculated based on the clinical usage of SBP (135 mg equivalent to 6 pills per day for adults with 60 kg in body weight) in Chinese population [23].
Three groups of CUMS-stressed mice randomly received vehicle alone (CUMS + vehicle), 20.25 mg/kg (CUMS + 20.25 mg/kg SBP) or 40.5 mg/kg SBP (CUMS + 40.5 mg/kg SBP) treatment for 6 weeks. The treatment was carried out in parallel with the 6 weeks of the CUMS procedure. An additional group of unstressed and untreated mice was included as control group for behavioral and biochemical examinations. Total 50 mice initially received CUMS procedures in a random manner (n = 20 for CUMS + vehicle, 20 for CUMS + 20.25 mg/kg SBP, and 10 for CUMS + 40.5 mg/kg SBP).
2.4. Behavioral tests
A sucrose preference test (SPT), a forced-swimming test (FST), and a tail suspension test (TST) were used to measure depressive behavior. An open field test (OFT) and an elevated plus maze (EPM) test were used to measure anxiety behavior. The behavioral procedures started at 3 days before the completion of the CUMS. The SPT was conducted on days 1 through 4 and the FST and TST on day 5. The OFT and EPM test were carried out on day 6.
2.4.1. Sucrose preference test
The SPT was conducted as described previously with minor modifications [24]. Briefly, to acclimatize sucrose preference, mice were exposed to two bottles containing 1% sucrose solution (w/v) with ad libitum access for 24 h in groups of three to five per cage. On day 2, one bottle containing 1% sucrose solution and another containing tap water were accessible for 24 h. On day 3, the positions of the two bottles were switched for another 24 h. At the end of the adaptation period, mice were deprived of food and water for 22 h. After that, the SPT was conducted in an individual mouse housed in a cage with free access to two respective bottles containing 1% sucrose solution and tap water for 2 h. To prevent side preference in drinking behavior, the position of the two bottles was switched in the middle of testing. Water and sucrose consumption were measured as changes in weight of fluid consumed. The sucrose preference was calculated from the formula: sucrose preference (%) = sucrose intake (g)/[sucrose intake (g) + water intake (g)] × 100%.
2.4.2. Forced-swimming test
The FST was conducted in a polycarbonate cylinder (30 cm in height and 20 cm in diameter) that was filled with water to a depth of 15 cm at 23 °C–25 °C, as reported previously. A mouse was placed in the cylinder for 6 min and its movement was recorded on videotape. The duration of immobility, defined as the absence of all movements except for motions required to maintain the head above the water, was obtained from the last 4 min of the trial with EthoVision XT7 software
2.4.3. Tail suspension test
The TST was performed in a specially manufactured tail suspension box as reported previously. Briefly, each mouse was suspended 50 cm above the floor of the box by fixing its tail tip (1 cm in length) with adhesive tape. The duration of immobility, defined as the absence of any movements of limbs and trunk except for whisker movement and respiration, was recorded on videotape over 6min of testing and analyzed with EthoVision XT7 software
2.4.4. Open field test
The OFT was conducted in a white square box (40 × 40 × 40 cm), in which the white floor is divided into the central zone (15 × 15 cm) and the surrounding zone. Each mouse was placed on the surrounding zone and allowed to explore freely for 5 min under white fluorescent light from above. Its movement trajectory in the box was recorded with video tracking software (Noldus Information Technology, Leesburg, VA, USA). The total distance moved was analyzed to evaluate locomotor activity. The time spent in and number of entries into the central zone were obtained to evaluate the extent of anxiety. The box was cleaned with 70% ethanol between tests
2.4.5. Elevated plus maze test
The EPM test was conducted in a roofless apparatus consisting of two open arms (35 × 5 cm) and two closed arms (35 × 5 × 15 cm), arranged such that the two arms of each type are opposite to each other. The maze is elevated to a height of 70 cm from the floor. The mouse was placed in the center area of the maze with its head directed toward an open arm and was then allowed to explore the maze freely for 5 min. The movement trajectory in the maze was recorded with video tracking software (Noldus Information Technology, Leesburg, VA, USA). Any subsequent visit to one of the four arms was counted when all four paws of a mouse entered. The time spent in and number of entries into the open arms were obtained to evaluate the extent of anxiety. The maze was cleaned with 70% ethanol between tests
2.5. Measurement of serum HPA-related hormones
After the behavioral tests, 0.5–0.6 mL of blood was collected from each mouse via cardiac puncture; serum was separated immediately and stored at −80 °C until assay. Corticotrophin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) levels were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Cloud-Clone Corp., Wuhan, China) according to the manufacturer’s instructions.
2.6. Measurement of brain monoamine neurotransmitters
After blood sampling via cardiac puncture, the mice were killed and their brains were rapidly removed. The hippocampus, striatum, and cerebral cortex were dissected, weighed, and stored at −80 °C until assay. The brain monoamine transmitters, norepinephrine (NE), dopamine (DA), and 5-hydroxytryptamine (5-HT), and their respective metabolites, 3-methoxy-4-hydroxyphenylglycol (MHPG), dihydroxyphenylacetic acid (DOPAC), and 5-hydroxyindole acetic acid (5-HIAA) were assayed using a high-performance liquid chromatography (HPLC) system with an electrochemical detector. Briefly, the tissues were homogenized with 100–150 μL of 0.1 M perchloric acid containing 0.1% ascorbic acid as antioxidant. The mixture was centrifuged at 12,000 rpm at 4 °C for 20 min, and 20 μL of the resultant supernatant was directly injected into an UltiMate 3000 UHPLC equipped with an UltiMate™ 3000 ECD-3000RS (Thermo Fisher Scientific, Waltham, MA, USA) on anACE Excel 2C18 column (100mm×2.1mm×1.7 μm) at the potential of 1000mV. The chromatographic mobile phase consisted of methanol (A) and 50 mmol/L sodium acetate (adjusted to pH 4.5 with acetic acid) (B), and the gradient program was developed as follows: maintain 99% B for 25 min, 99.5% B for 25–40 min, and keep 99% B for 20 min for equilibrium. The flow rate was kept at 0.1 mL/min. The contents of transmitters, metabolites and turnover rate (metabolite/transmitter) were obtained from each brain region.
2.7. Western blot analysis
After blood sampling via cardiac puncture, the mice were decapitated. The brains were removed, and the hippocampus and cerebral cortex were rapidly dissected for Western Blot analysis. The three neurotrophic factors, brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and glial cell-derived neurotrophic factor (GDNF), were examined in the two brain regions. Briefly, the hippocampus and cerebral cortex were homogenized in radio-immunoprecipitation assay buffer (RIPA Buffer, Sigma Aldrich, USA) containing 1% protease inhibitor cocktail and 2% phenylmethanesulfonyl fluoride (PMSF; Sigma–Aldrich, USA) at 4 °C for 30 min. All tissues were centrifuged at 13,000 rpm for 20 min. The supernatant was collected and diluted appropriately; the diluent was mixed with Bio-Rad protein assay dye reagent concentrate (Bio-Rad Laboratories Inc.). The absorbance of the mixture was detected with a micro-plate reader (Bio-Rad Laboratories, Inc.) at 595 nm. The final protein concentration was calculated based on the standard curve generated by bovine serum albumin (BSA; Calbiochem, USA). The lysate was mixed with 6 × loading buffer and boiled at 99 °C for 8 min for denaturation. After that, the samples were stored at −20 °C for further use.
Twenty micrograms of protein were separated using 10%–12% SDS-PAGE in running buffer (25 mM Tris, 190 mM glycine, 0.1% SDS) at 100 V for 2 h and transferred electrophoretically onto polyvinylidene difluoride membranes (PVDF; 0.22 μM; Bio-Rad Laboratories, Inc.) in transfer buffer (25 mM Tris, 190 mM glycine, 20% methanol) at 100 V at 4 °C for 2 h. Nonspecific binding sites were blocked by 5% BSA dissolved in Tris-buffered saline Tween-20 (TBST; 20 mM Tris, 150 mM NaCl, 0.1% Tween-20, pH 7.4) for 4 h at 4 °C. The transferred membranes were then blotted with the primary antibodies, rabbit anti-BDNF (1:1000, Santa Cruz Biotechnology, USA), rabbit anti-NGF (1:1000, Abcam, Cambridge, USA), and rabbit anti-GDNF (1:1000, Abcam), and mouse anti-GAPDH (1:5000, Immunoway, USA) at 4 °C overnight. After rinsing with TBST, the membranes were incubated with suitable secondary antibodies (1:2000, Santa Cruz Biotechnology, USA) at 4 °C for 4 h. Chemiluminescence was detected using an enhanced chemiluminescence detection kit (GE Healthcare, UK). The intensity of the bands was quantified by scanning densitometry using Image Lab 5.1 software (Bio-Rad, Laboratories, Inc.). The mean value of the intensity was obtained from at least three independent experiments.
2.8. Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM) and analyzed with one-way analysis of variance (ANOVA), followed by post hoc Dunnett’s test with GraphPad Prism 7.0 software (La Jolla, CA). The results were considered statistically significant if the P value was less than 0.05.
3. Results
3.1. Effects of SBP on depression-like behavior
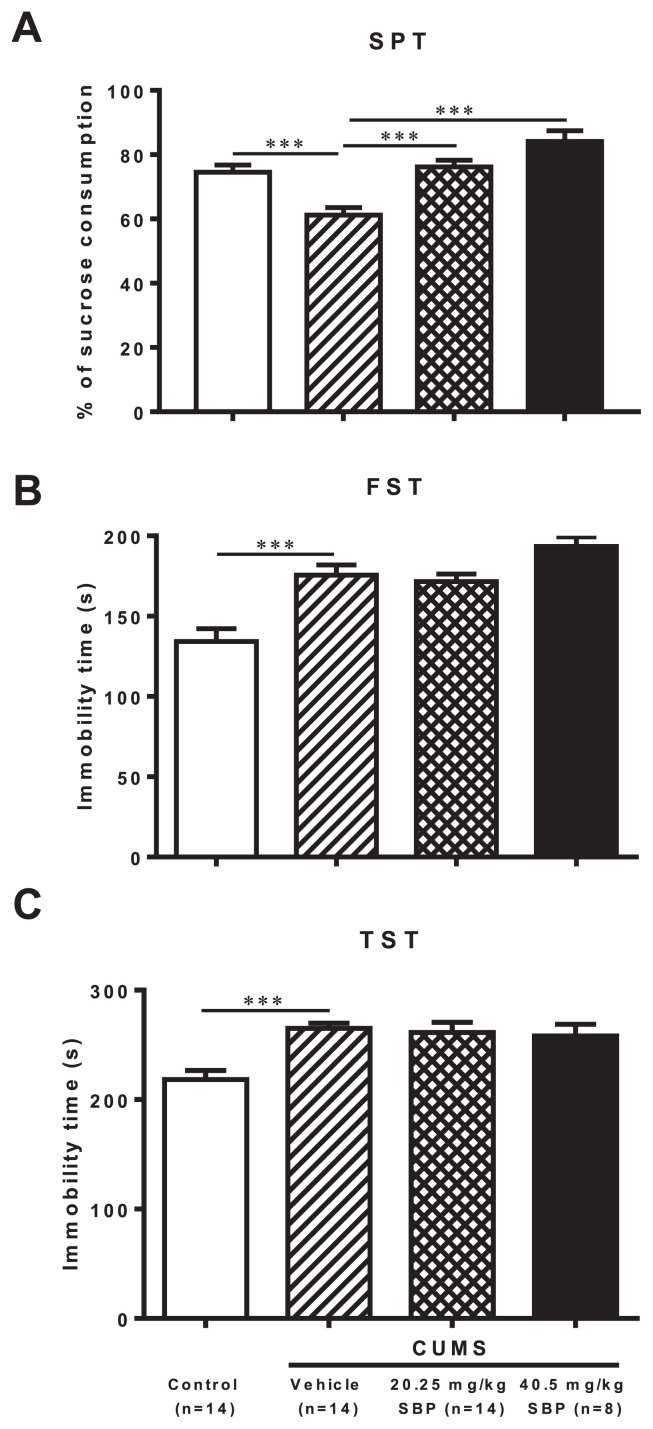
ANOVA revealed significant group effects on the three behavioral paradigms of depression, SPT (F3,46 = 14.390, P < 0.0001), FST (F3,46 = 13.500, P < 0.0001), and TST (F3,46 = 7.972, P = 0.0002) (Fig. 1). CUMS-stressed mice showed a significant decrease in sucrose consumption (P = 0.0003) in the SPT and a marked increase in immobility time spent in the FST (P = 0.0001) and in the TST (P = 0.0002) compared with controls. The stressed mice treated with both doses of SBP consumed significantly more sucrose (P = 0.0001) but showed no significant effects on immobility time spent in the FST and TST compared with stressed mice treated with vehicle.
Fig. 1.
The effects of the SBP on chronic unpredictable mild stress (CUMS)-induced depression-like behavior in sucrose preference test (SPT) (A), forced-swimming test (FST) (B), and tail suspension test (TST) (C). Data are expressed as mean ± SEM (n = 8–14) and examined with one-way analysis of variance (ANOVA), followed by post hoc Dunnett’s test: ***P < 0.001 compared with Vehicle + CUMS group.
3.2. Effects of SBP on anxiety-like behavior
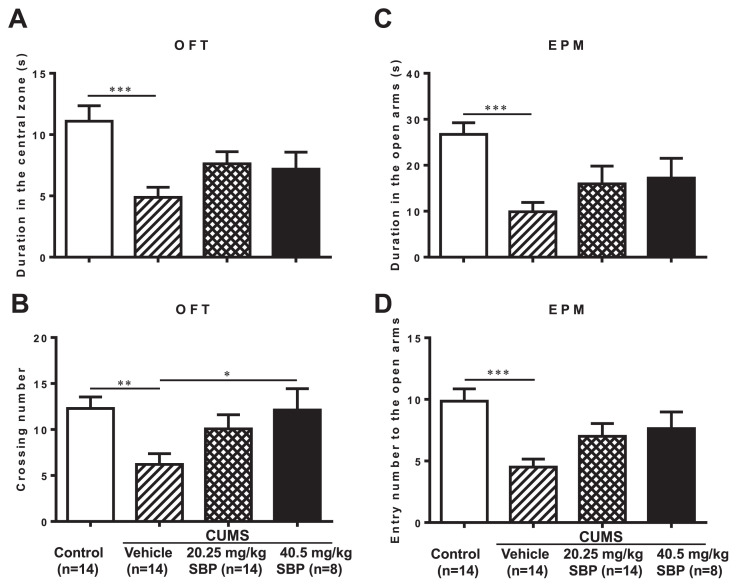
Significant differences among groups were observed in the OFT in duration spent in the central zone (F3,46 = 6.054, P = 0.0015) and the number of entries into the central zone (F3,46 = 3.785, P = 0.0165), and in the EPM test duration spent in (F3,46 = 5.490, P = 0.0026) and number of entries into (F3,46 = 5.506, P = 0.0026) the open arms (Fig. 2). The stressed mice receiving the higher dose (40.5 mg/kg) of SBP had markedly more entries into the central zone in the OFT than those that received vehicle (P = 0.0373). Either two doses of SBP did not affect the duration spent in or the number of entries into the open arms of the EPM.
Fig. 2.
The effects of the SBP on chronic unpredictable mild stress (CUMS)-induced anxiety-like behavior in open field test (OFT) and elevated plus maze (EPM) test. (A) Duration in the central zone in the OPT; (B) number of entries into the central zone in the OFT; (C) Duration in the open arms in EPM; (D) number of entries into the open arms in EPM. Data are expressed as mean ± SEM (n = 8–14) and examined with one-way analysis of variance (ANOVA), followed by post hoc Dunnett’s test: *P < 0.05, **P < 0.01, ***P < 0.001 compared with Vehicle + CUMS group.
3.3. Effects of SBP on serum levels of CRH and ACTH
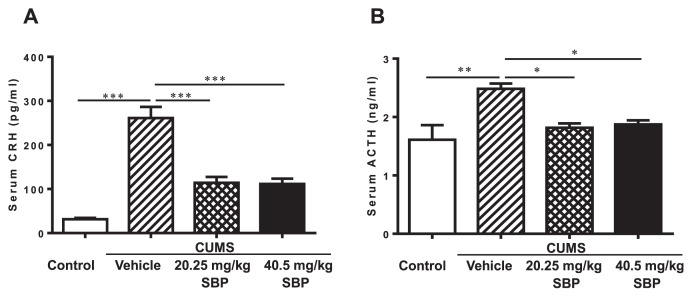
Significant main effects of groups were observed on CRH (F3,12 = 37.170, P < 0.0001) and ACTH (F3,12 = 6.937, P = 0.0058) (Fig. 3). The stressed mice had a striking elevation of serum levels of the two hormones compared with the unstressed mice (P ≤ 0.0027). Both doses of SBP significantly suppressed elevated levels of the two hormones compared with vehicle treatment of stressed mice (P ≤ 0.0270).
Fig. 3.
The effects of the SBP on serum levels of corticotrophin-releasing hormone (CRH) (A) and adrenocorticotropic hormone (ACTH) (B) in mice with chronic unpredictable mild stress (CUMS). Data are expressed as mean ± SEM (n = 4) and examined with one-way analysis of variance (ANOVA), followed by post hoc Dunnett’s test: *P < 0.05, **P < 0.01, ***P < 0.001 compared with Vehicle + CUMS group.
3.4. Effects of SBP on levels of brain neurotransmitters and related metabolites
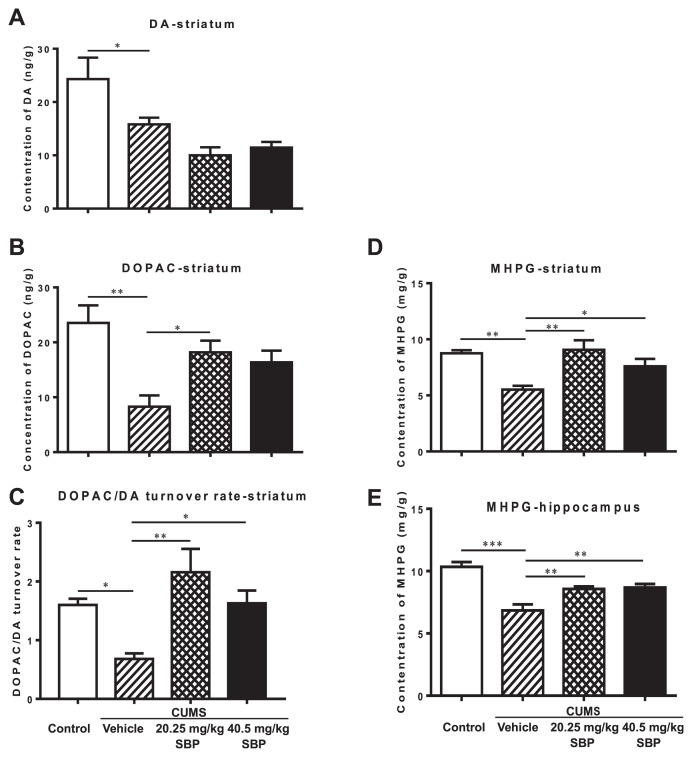
The representational chromatograms are shown in Fig. S1. Methodological limitations precluded detections of NE, 5-HT, and its metabolite 5-HIAA in the brain regions examined. Significant main effects of groups were detected in hippocampal MHPG (F3,14 = 15.810, P < 0.0001), striatal MHPG (F3,18 = 8.044, P = 0.0013), DA (F3,20 = 7.836, P = 0.0012), DOPAC (F3,17 = 6.629, P = 0.0036), and striatal DOPAC/DA turnover rate (F3,16 = 6.687, P = 0.0039) (Fig. 4). Post-hoc analyses further revealed that the monoamines and metabolite levels and DOPAC/DA turnover ratio of the stressed group were significantly lower than those of the unstressed group (P ≤ 0.0429). Both doses of SBP remarkably restored hippocampal and striatal MHPG levels (P ≤ 0.0390) and striatal DOPAC/DA turnover ratio (P ≤ 0.0316). The striatal DOPAC levels of the group treated with the lower dose (20.25 mg/kg) of SBP was approximately twofold higher than those of the vehicle treated stressed group (P = 0.0286).
Fig. 4.
The effects of the SBP on the contents of monoaminergic neurotransmitters and their metabolites in the hippocampus and striatum of mice with chronic unpredictable mild stress (CUMS). The left column indicates striatal dopamine (DA) (A), its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) (B), and the turnover ratio (C). The right column represents 3-methoxy-4-hydroxyphenylglycol (MHPG), a metabolite of norepinephrine degradation in the striatum (D) and hippocampus (E). Data are expressed as mean ± SEM (n = 4–6) and examined with one-way analysis of variance (ANOVA), followed by post hoc Dunnett’s test: *P < 0.05, **P < 0.01, ***P < 0.001 compared with Vehicle + CUMS group.
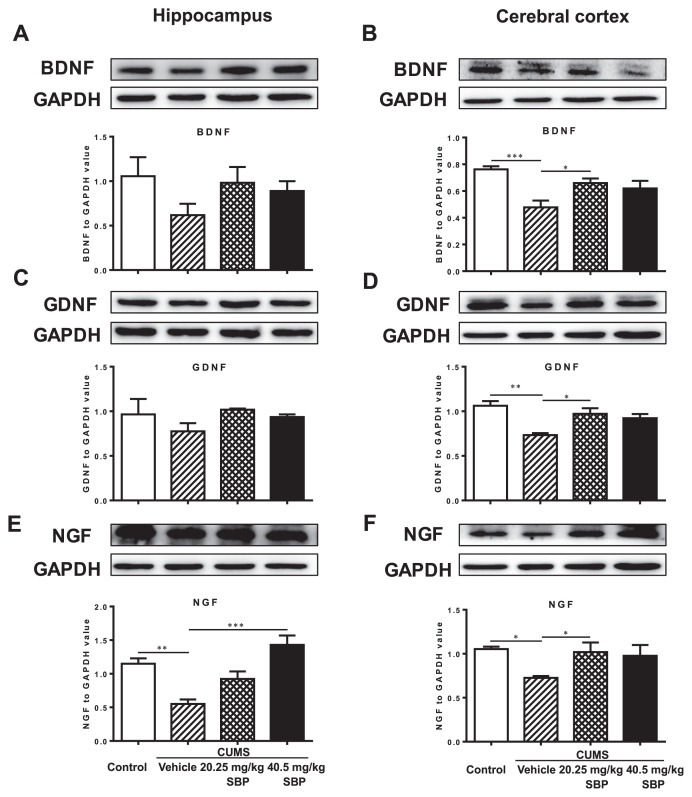
3.5. Effects of SBP on the three neurotrophins of brain regions
Significant main effects of groups were observed on cortical BDNF (F3,15 = 8.283, P = 0.0017), GDNF (F3,10 = 8.442, P = 0.0043), NGF (F3,14 = 4.736, P = 0.0175), and hippocampal NGF (F3,8 = 12.820, P = 0.0020), but not hippocampal BDNF (F3,8 = 1.395, P = 0.3132) and GDNF (F3,9 = 1.259, P = 0.3453) (Fig. 5). The stressed mice displayed markedly lower expression levels of the three neurotrophic factors in the cortex (P ≤ 0.0166) and of hippocampal NGF (P = 0.0090). The stressed mice treated with 20.25 mg/kg SBP had significantly higher expression levels of cortical BDNF, GDNF, and GNF than did the vehicle treated stressed mice (P ≤ 0.0222). The expression level of the hippocampal NGF of 40.5 mg/kg SBP-treated mice was also significantly greater than that of the vehicle treated stressed mice (P = 0.0009).
Fig. 5.
The effects of the SBP on the three neurotrophins, brain-derived neurotrophic factor (BDNF) (A, B), glial cell-derived neurotrophic factor (GDNF) (C, D), and nerve growth factor (NGF) (E, F), in the hippocampus (left column) and cerebral cortex (right column). Plots C and E share the same glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control bands and plots D and F share the same GAPDH control bands. Data are expressed as mean ± SEM (n = 3–6) and examined with one-way analysis of variance (ANOVA), followed by post hoc Dunnett’s test: *P < 0.05, **P < 0.01, ***P < 0.001 compared with Vehicle + CUMS group.
4. Discussion
Similar to previous studies [20], this study showed that the exposure of mice to the CUMS procedure for 6 weeks caused diminished sucrose intake in the SPT, increased immobility time in the FST and TST, shorter duration remaining in and fewer entries into the central zone of OFT and into the open arms of the EPM. The CUMS also induced striking elevations of blood CRH and ACTH, the two key hormones of the HPA axis, confirming the notion that chronic stress results in HPA axis hyperactivity, leading to the increased levels of related hormones [25]. This study well validated CUMS-induced depressive and anxiety behavior inmultiple paradigms and HPA axis hyperactivity.
This study revealed that chronic treatment with SBP at both doses restored sucrose intake in the SPT; the high dose also increased the number of entries into the central zone in the OFT apparatus, clearly indicating antidepressant- and anxiolytic-like effects of SBP. Nevertheless, neither dose had an effect on the other two depressive paradigms (FST and TST). These findings are highly consistent with previous studies that have confirmed that CUMS is more likely to induce “anhedonic” behavior, rather than “desperate” behavior [26]. Anhedonia represents a mild depressive status that is more sensitively detected in the SPT than other depressive paradigms, whereas “desperate” behavior is often caused by severe acute stressful events and is indicative of passive immobility in the FST and TST [26]. It therefore appears that SBP particularly suited to treat stress-induced mild mood and anxiety disorders.
This study also found that both doses of SBP strikingly lowered CUMS-induced elevation of blood CRH and ACTH levels. It is well documented that dysregulation of the HPA axis with elevated CRH and ACTH levels is extensively involved in the etiopathogenesis of depression and anxiety-related disorders [27–31]. Our results suggest an association of the antidepressant- and anxiolytic-like effects of SBP with the suppression of HPA axis hyperactivity.
Central monoaminergic systems play an essential role in the pathogenesis of mood and anxiety disorders [32]. A large body of evidences have confirmed a close association of the pathophysiological mechanisms of depression and anxiety with dysregulation of serotonergic, noradrenergic, dopaminergic, glutamatergic and GABAergic transmission system [33,34]. In this study, although the methodological limitations prevented us from detecting the contents of NE, 5-HT, and its metabolite 5-HIAA in the brain regions examined, we showed that CUMS produced a widespread decrease in the striatal DA, its principal metabolite DOPAC and its turnover rate, and the striatal and hippocampal MHPG, a key metabolite of NE. Similar results have also been observed in CUMS-induced depression in rats [35]. Moreover, this study revealed that long-term treatment with SBP at both doses reversed the stress-induced decrease in hippocampal and striatal MHPG levels and striatal DOPAC/DA turnover ratio. The low dose of SBP additionally restored the stress-induced reduction in the striatal DOPAC. It has been demonstrated that anhedonic behavior is partly caused by reduced function of the mesostriatal and mesolimbic DA systems, which play an essential role in endogenous reward mechanisms and stress reduction [36]. Rats that display CUMS-induced anhedonic behavior showed greater sensitivity to NE and elevated expression of neuronal NE transporter [37]. These results suggest that the effects of SBP observed in reducing anhedonia- and anxiety-like symptoms appear to be associated with its modulation of multiple central catecholamine systems by preventing the stress-induced abnormalities in the metabolism of brain monoamine transmitters.
BDNF, GDNF, and NGF are the three most abundant neurotrophins that are heavily involved in the pathophysiology of mood and other stress-related disorder [38]. The alterations in neurotrophins levels are associated with neuronal death, survival and dendritic retraction [39]. Clinical studies have shown the decreased neurotrophin levels in patients with suicide victims [40]. Furthermore, anxiety has been confirmed to correlate with anatomical changes related to the BDNF polymorphism [41]. In this study, we revealed that CUMS caused a widespread decrease in the expression levels of the three neurotrophins in the cortex. This is in agreement with results obtained from the postmortem brain of depressed patients with MDD, in which significant reduction was observed in mRNA and protein levels of BDNF and other several neurotrophic factors in critical brain regions, including the prefrontal cortex and hippocampus [42]. However, the present study did not detect the downregulation of hippocampal BDNF and GDNF. One recent study also has shown that different types of stress produced differences in the expression of multiple neuroplasticity markers in the prefrontal cortex and hippocampus [43]. It seems that different neurotrophins may have differential patterns and brain region specificity in response to different types of stress.
This study further revealed that chronic treatment with SBP, in either low or high doses, reversed the CUMS-induced decrease of the expression levels of the three neurotrophins in the cortex and NGF in the hippocampus. This finding is in line with previous studies, confirming that SBP has significant effects in enhancing vascular endothelial growth factor (VEGF) protein expression in the heart [7]. Antidepressant treatment increased peripheral and brain BDNF levels in patients with MDD [44,45]. The antidepressant efficacy has been shown to be associated with the regulation of the synthesis and release of related neurotrophins in the hippocampus and cortex [44,45]. Thus, our study suggests that SBP could prevent stress-induced aberrant alternation in brain neurotrophins; this preventive activity is an important mechanism in the antidepressant and anxiolytic effects of SBP.
Several limitations of this study should be noted. First, this study did not include a conventional drug that possesses dual antidepressant and anxiolytic effects as positive control. Whether the antidepressant and anxiolytic potency of SBP is comparable to that of conventional drugs could not be determined. Second, we only tested SBP as a whole preparation, but did not examine individual materials and major constituents that may exert dominant effects, although it is postulated that Radix Ginseng, Cortex Cinnamomi, and Styrax may play the principle role in the psychotropic effects of SBP. Novel antidepressant and anxiolytic compounds existing in SBP deserve further identification. Finally, there are no clinical studies reported about the use of SBP in patients with mental diseases. Its clinical benefits and risks in the prevention of depressive and anxiety disorders need further evaluation in clinical settings.
5. Conclusions
In summary, this study suggests that SBP could prevent and alleviate prolonged stress-induced anhedonia and anxiety in association with its suppression of the hypothalamic-pituitary-adrenal (HPA) axis hyperactivity, modulation of brain monoamine neurotransmitter metabolism and neurotrophins. SBP may be particularly suitable for the management of depressive and anxiety disorders in patients with cardiac conditions.
Acknowledgments
This work was supported by Shanghai Hutchison Pharmaceuticals and General Research Fund (GRF) of Research Grant Council of HKSAR (17115017).
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2018.08.001.
Funding Statement
This work was supported by Shanghai Hutchison Pharmaceuticals and General Research Fund (GRF) of Research Grant Council of HKSAR (17115017).
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
REFERENCES
- 1. Dickens C. Depression in people with coronary heart disease: prognostic significance and mechanisms. Curr Cardiol Rep. 2015;17(10):83. doi: 10.1007/s11886-015-0640-6. [DOI] [PubMed] [Google Scholar]
- 2. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365–72. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 3. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 4. Grippo AJ. Mechanisms underlying altered mood and cardiovascular dysfunction: the value of neurobiological and behavioral research with animal models. Neurosci Biobehav Rev. 2009;33(2):171–80. doi: 10.1016/j.neubiorev.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PCo China. Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Press; 2015. [Google Scholar]
- 6. Zhou Z, Shen WX, Yu LL, Xu C, Wu QB. A Chinese patent medicine, Shexiang Baoxin Pill, for Non-ST-elevation acute coronary syndromes: a systematic review. J Ethnopharmacol. 2016;194:1130–9. doi: 10.1016/j.jep.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 7. Zhang KJ, Zhu JZ, Bao XY, Zheng Q, Zheng GQ, Wang Y. Shexiang Baoxin pills for coronary heart disease in animal models: preclinical evidence and promoting angiogenesis mechanism. Front Pharmacol. 2017;8:404. doi: 10.3389/fphar.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang BY. Contemporary pharmacology of Chinese medicine. Tianjin: Tianjin Science & Technology Press; 1999. [Google Scholar]
- 9. Meng Y, Xiao Q, Bai JY, Xiao X, Zhang S, Zhu XY, et al. Resolution and chiral recognition of muscone as well as actions on neural system. J Asian Nat Prod Res. 2014;16(12):1166–70. doi: 10.1080/10286020.2014.996141. [DOI] [PubMed] [Google Scholar]
- 10. Yao Y, Huang HY, Yang YX, Guo JY. Cinnamic aldehyde treatment alleviates chronic unexpected stress-induced depressive-like behaviors via targeting cyclooxygenase-2 in mid-aged rats. J Ethnopharmacol. 2015;162:97–103. doi: 10.1016/j.jep.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 11. Bahramsoltani R, Farzaei MH, Farahani MS, Rahimi R. Phytochemical constituents as future antidepressants: a comprehensive review. Rev Neurosci. 2015;26(6):699–719. doi: 10.1515/revneuro-2015-0009. [DOI] [PubMed] [Google Scholar]
- 12. Cui J, Jiang L, Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacol. 2012;26(5):697–713. doi: 10.1177/0269881111415735. [DOI] [PubMed] [Google Scholar]
- 13. Fajemiroye JO, Polepally PR, Chaurasiya ND, Tekwani BL, Zjawiony JK, Costa EA. Oleanolic acid acrylate elicits antidepressant-like effect mediated by 5-HT1A receptor. Sci Rep. 2015;5:11582. doi: 10.1038/srep11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ho TJ, Hung CC, Shih TL, Yiin LM, Chen HP. Investigation of borneols sold in Taiwan by chiral gas chromatography. J Food Drug Anal. 2018;26(1):348–52. doi: 10.1016/j.jfda.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang QL, Fu BM, Zhang ZJ. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood-brain barrier permeability. Drug Deliv. 2017;24(1):1037–44. doi: 10.1080/10717544.2017.1346002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang P, Fu P, Xiang L, Wang S, Liu X, Yang L, et al. The effectiveness of borneol on pharmacokinetics changes of four ginsenosides in Shexiang Baoxin Pill in vivo. Biomed Chromatogr. 2014;28(3):419–27. doi: 10.1002/bmc.3037. [DOI] [PubMed] [Google Scholar]
- 17. Lei K, He GF, Zhang CL, Liu YN, Li J, He GZ, et al. Investigation of the synergistic effects of haloperidol combined with Calculus Bovis Sativus in treating MK-801-induced schizophrenia in rats. Exp Anim. 2018;67(2):163–73. doi: 10.1538/expanim.17-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Z, Gong X, Lu Y, Du S, Yang Z, Bai J, et al. Enhancing effect of borneol and muscone on geniposide transport across the human nasal epithelial cell monolayer. PLoS One. 2014;9(7):e101414. doi: 10.1371/journal.pone.0101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang M, Du Y, Li W, Yin X, Yang N, Qie A, et al. SuHeXiang essential oil inhalation produces antidepressant- and anxiolytic-like effects in adult mice. Biol Pharm Bull. 2018;41(7):1040–8. doi: 10.1248/bpb.b18-00082. [DOI] [PubMed] [Google Scholar]
- 20. Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carnevali L, Montano N, Statello R, Sgoifo A. Rodent models of depression-cardiovascular comorbidity: bridging the known to the new. Neurosci Biobehav Rev. 2017;76:144–53. doi: 10.1016/j.neubiorev.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 22. Zhu S, Wang J, Zhang Y, Li V, Kong J, He J, et al. Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res. 2014;1576:81–90. doi: 10.1016/j.brainres.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Wei W, Wu XM, Li YJ. Experimental methodology of pharmacology. 4th ed. Beijing: People’s Medical Publishing House; 2010. [Google Scholar]
- 24. Rao C, Shi H, Zhou C, Zhu D, Zhao M, Wang Z, et al. Hypothalamic proteomic analysis reveals dysregulation of glutamate balance and energy metabolism in a mouse model of chronic mild stress-induced depression. Neurochem Res. 2016;41(9):2443–56. doi: 10.1007/s11064-016-1957-2. [DOI] [PubMed] [Google Scholar]
- 25. Cai L, Li R, Tang WJ, Meng G, Hu XY, Wu TN. Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus-pituitary-adrenal axis. Eur Neuropsychopharmacol. 2015;25(8):1332–41. doi: 10.1016/j.euroneuro.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 26. Stepanichev MY, Tishkina AO, Novikova MR, Levshina IP, Freiman SV, Onufriev MV, et al. Anhedonia but not passive floating is an indicator of depressive-like behavior in two chronic stress paradigms. Acta Neurobiol Exp. 2016;76(4):324–33. doi: 10.21307/ane-2017-031. [DOI] [PubMed] [Google Scholar]
- 27. Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, Jr, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatr. 2017;22(4):527–36. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schatzberg AF. Anna-Monika Award Lecture, DGPPN Kongress, 2013: the role of the hypothalamic-pituitary-adrenal (HPA) axis in the pathogenesis of psychotic major depression. World J Biol Psychiatr. 2015;16(1):2–11. doi: 10.3109/15622975.2014.916414. [DOI] [PubMed] [Google Scholar]
- 29. Sher L, Oquendo MA, Burke AK, Cooper TB, Mann JJ. Combined dexamethasone suppression-corticotrophin-releasing hormone stimulation test in medication-free major depression and healthy volunteers. J Affect Disord. 2013;151(3):1108–12. doi: 10.1016/j.jad.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 30. Nothdurfter C, Schmotz C, Sarubin N, Baghai TC, Laenger A, Lieb M, et al. Effects of escitalopram/quetiapine combination therapy versus escitalopram monotherapy on hypothalamic-pituitary-adrenal-axis activity in relation to antidepressant effectiveness. J Psychiatr Res. 2014;52:15–20. doi: 10.1016/j.jpsychires.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 31. Belvederi Murri M, Pariante C, Mondelli V, Masotti M, Atti AR, Mellacqua Z, et al. HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology. 2014;41:46–62. doi: 10.1016/j.psyneuen.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 32. Tafet GE, Nemeroff CB. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28(2):77–88. doi: 10.1176/appi.neuropsych.15030053. [DOI] [PubMed] [Google Scholar]
- 33. Liu L, Liu C, Wang Y, Wang P, Li Y, Li B. Herbal medicine for anxiety, depression and insomnia. Curr Neuropharmacol. 2015;13(4):481–93. doi: 10.2174/1570159X1304150831122734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feng LY, Battulga A, Han E, Chung H, Li JH. New psychoactive substances of natural origin: a brief review. J Food Drug Anal. 2017;25(3):461–71. doi: 10.1016/j.jfda.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu P, Wang KZ, Lu C, Dong LM, Le Zhai J, Liao YH, et al. Antidepressant-like effects and cognitive enhancement of the total phenols extract of Hemerocallis citrina Baroni in chronic unpredictable mild stress rats and its related mechanism. J Ethnopharmacol. 2016;194:819–26. doi: 10.1016/j.jep.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 36. Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep. 2015;2(3):146–53. doi: 10.1007/s40473-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouzinova EV, Møller-Nielsen N, Boedtkjer DB, Broegger T, Wiborg O, Aalkjaer C, et al. Chronic mild stress-induced depression-like symptoms in rats and abnormalities in catecholamine uptake in small arteries. Psychosom Med. 2012;74(3):278–87. doi: 10.1097/PSY.0b013e31824c40a9. [DOI] [PubMed] [Google Scholar]
- 38. Castren E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. 2017;97:119–26. doi: 10.1016/j.nbd.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 39. Haase J, Brown E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression–a central role for the serotonin transporter? Pharmacol Ther. 2015;147:1–11. doi: 10.1016/j.pharmthera.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 40. Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N. Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. BioMed Res Int. 2014;2014:932757. doi: 10.1155/2014/932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Domingos da Silveira da Luz AC, Pereira Dias G, do Nascimento Bevilaqua MC, Cocks G, Gardino PF, Thuret S, et al. Translational findings on brain-derived neurotrophic factor and anxiety: contributions from basic research to clinical practice. Neuropsychobiology. 2013;68(3):129–38. doi: 10.1159/000353269. [DOI] [PubMed] [Google Scholar]
- 42. Sharma AN, da Costae Silva BF, Soares JC, Carvalho AF, Quevedo J. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: a comprehensive review of human studies. J Affect Disord. 2016;197:9–20. doi: 10.1016/j.jad.2016.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kucharczyk M, Kurek A, Pomierny B, Detka J, Papp M, Tota K, et al. The reduced level of growth factors in an animal model of depression is accompanied by regulated necrosis in the frontal cortex but not in the hippocampus. Psychoneuroendocrinology. 2018;94:121–33. doi: 10.1016/j.psyneuen.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 44. Zhou C, Zhong J, Zou B, Fang L, Chen J, Deng X, et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS One. 2017;12(2):e0172270. doi: 10.1371/journal.pone.0172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amidfar M, Réus GZ, Quevedo J, Kim YK, Arbabi M. Effect of co-administration of memantine and sertraline on the antidepressant-like activity and brain-derived neurotrophic factor (BDNF) levels in the rat brain. Brain Res Bull. 2017;128:29–33. doi: 10.1016/j.brainresbull.2016.11.003. [DOI] [PubMed] [Google Scholar]