Abstract
MK‐8507 is an investigational HIV‐1 nonnucleoside reverse transcriptase inhibitor being developed for the treatment of HIV‐1 infection. MK‐8507 contains 2 trifluoromethyl groups that may result in fluoride release through metabolism, but the extent of MK‐8507–related fluoride release in humans has yet to be determined. This double‐blind, placebo‐controlled, 2‐period, parallel‐group, multiple‐dose trial in healthy participants without HIV‐1 who were administered a fluoride‐restricted diet and once‐weekly doses of MK‐8507 aimed to estimate the relationship between MK‐8507 dose and fluoride exposure. A total of 15 adult male and 3 adult female (of non‐childbearing potential) participants were randomized to receive MK‐8507 200 mg (n = 6), MK‐8507 800 mg (n = 6), or placebo (n = 6). Change from baseline in mean daily fluoride excretion averaged over 7 days following the administration of MK‐8507 200 mg resulted in a net mean increase of 19.8 μmol (90% confidence interval, 12.2‐27.4) relative to placebo and did not exceed 57 μmol, a threshold related to the mean difference between the daily reference dose set by the US Environmental Protection Agency and the average dietary fluoride intake in the United States. However, daily urinary fluoride excretion exceeded the threshold following administration of 800 mg MK‐8507 (75.1 μmol [90% confidence interval, 67.5‐82.7]). Assuming a linear relationship between MK‐8507 dose and estimated mean daily fluoride released at steady‐state, data interpolation suggests that the US Environmental Protection Agency reference dose for fluoride would not be exceeded in most patients when administering MK‐8507 at doses currently under clinical investigation (≤400 mg once weekly).
Keywords: fluoride, human immunodeficiency virus, MK‐8507, nonnucleoside reverse transcriptase inhibitor
MK‐8507 is an investigational HIV‐1 nonnucleoside reverse transcriptase inhibitor being developed for the treatment of HIV‐1 infection. Preclinical and clinical data have demonstrated that MK‐8507 has a favorable safety and tolerability profile and pharmacokinetic (PK) properties that support once‐weekly administration. 1 In particular, PK studies indicate that steady‐state plasma concentrations of MK‐8507 are reached after the first week of administration. 2 Of note, MK‐8507 contains 2 trifluoromethyl groups that may result in fluoride release via metabolism, but the relevance of this pathway and the extent of MK‐8507–related fluoride release in humans has yet to be determined.
At appropriate levels, fluoride is beneficial to teeth, preventing dental caries. 3 Although high levels of fluoride can be problematic, acute fluoride toxicity is uncommon in adults, generally occurring only when fluoride intake exceeds 5 mg/kg. 4 Symptoms of fluoride toxicity can present within minutes and tend to be predominantly gastrointestinal, although neurologic and cardiovascular effects can also occur. 4 Chronic fluoride toxicity (long‐term exposure >6 mg/d) is associated with dental fluorosis in children and skeletal fluorosis in adults, which results in increased bone density. 5 , 6 Bones affected by skeletal fluorosis exhibit greater fragility, which carries an elevated risk of fractures. 5 , 6 Furthermore, ligaments and tendons can also become calcified in individuals with skeletal fluorosis, reducing mobility. 5
To reduce dental fluorosis and systemic toxicity, the US Environmental Protection Agency recommends a maximum daily intake (or reference dose [RfD]) of 0.08 mg/kg of fluoride per day (equal to 4.8 mg/d for a person weighing 60 kg), defined as “an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime.” 7 A person living in the United States consumes on average 2.91 mg of fluoride a day, 7 suggesting that consumption of an additional 1.89 mg of fluoride each day can be accommodated without exceeding the maximum recommended daily intake level. However, average fluoride consumption is highly variable from region to region due to differences in diet and natural fluoride levels in water 8 ; therefore, there is a level of uncertainty regarding an acceptable amount of additional fluoride consumption.
MK‐8507 is being investigated at doses of 100, 200, and 400 mg once weekly in combination with the nucleoside reverse transcriptase translocation inhibitor islatravir (20 mg) for the treatment of HIV‐1 infection (ClinicalTrials.gov ID: NCT04564547). It is therefore important to understand the degree of fluoride exposure that may result from MK‐8507 metabolism at clinically relevant doses. This trial was conducted to estimate the relationship between MK‐8507 dose and fluoride exposure in individuals not infected with HIV‐1 administered a fluoride‐restricted diet and once‐weekly doses of MK‐8507.
Methods
A randomized, double‐blind, placebo‐controlled, 2‐period, parallel‐group, multiple‐dose trial was performed at a single center in Lincoln, Nebraska. The trial protocol was reviewed and approved by Chesapeake Institutional Review Board (Columbia, Maryland) before commencement. The trial was conducted in accordance with the Good Clinical Practice and International Council for Harmonization guidelines. All participants provided written informed consent before enrollment in the study in accordance with the US Food and Drug Administration Code of Federal Regulations, Title 21, Part 50, Protection of Human Subjects.
Trial Design
The trial design is depicted in Figure 1. Participants were enrolled in 2 cohorts of 9 participants each. No drug was administered during the 6‐day run‐in period (period 1). On completion of period 1, all participants entered period 2 directly, during which cohort A was randomized 2:1 to receive once‐weekly oral doses of MK‐8507 200 mg (theoretical maximum fluoride exposure due to full metabolism of MK‐8507 through this pathway calculated at 46.4 mg or 2440 μmol) or placebo on days 1, 8, and 15; and cohort B was randomized 2:1 to receive once‐weekly oral doses of MK‐8507 800 mg (theoretical maximum fluoride exposure due to metabolism of MK‐8507 through this pathway calculated at 185.4 mg or 9760 μmol) or placebo on days 1, 8, and 15.
Figure 1.
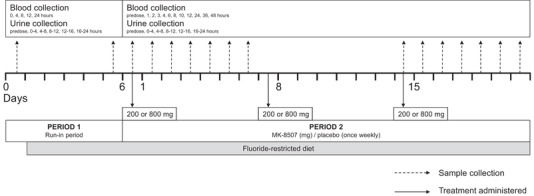
Trial design. Treatment regimen is indicated by dashed arrows, and sample (blood and urine) collection is indicated by solid arrows.
Participants consumed an unrestricted diet, including water, and used their usual dental products to gain an estimate of “constitutive” fluoride intake on day 1 of period 1. Participants consumed a standardized fluoride‐restricted diet from day 2 of period 1 until completion of the trial, limited to a total daily dietary intake of <0.4 mg of fluoride, during which consumption of all fluoride‐containing products (eg, tap water, dietary supplements, fluoride‐containing toothpaste, tea) was restricted.
To ensure adequate urine collection for fluoride analysis, 240 mL of defluoridated water was administered at the time of dosing and at least a further 200 mL was administered at 1, 3, and 6 hours after dosing. Additional defluoridated water was administered ad libitum. During period 1, all participants were domiciled in the clinical research unit.
Eligibility Criteria
Eligible participants were adult men or women of non‐childbearing potential aged 21 to 55 years and weight of >40 kg with a body mass index of ≤35.0 kg/m2. Calcium, magnesium, phosphorus, and parathyroid hormone levels were required to be within normal limits and an estimated creatinine clearance ≥90 mL/min based on the Cockcroft‐Gault equation was additionally required. Participants must not have had any clinically significant medical history. Intolerance to galactose, lactase deficiency, glucose‐galactose malabsorption, significant multiple and/or severe allergies, or anaphylactic reaction or significant intolerability to prescription or nonprescription drugs or food were also exclusionary. Participants must also not have had a positive screening result for HIV, hepatitis B surface antigen, or hepatitis C virus.
Urine and Plasma Sampling and Fluoride PK Analyses
Urine samples for the determination of fluoride excretion were collected over 0 to 4, 4 to 8, 8 to 12, 12 to 16, and 16 to 24‐hour index time intervals on days 1 and 6 in period 1. In period 2, urine samples were collected immediately before dosing and over 0 to 4, 4 to 8, 8 to 12, 12 to 16, and 16 to 24‐hour intervals after dosing on days 1, 2, 3, 15, 16, and 17. Additionally, urine was collected over 24 hours on days 4 to 7 and 18 to 21 in period 2. The amount of fluoride excreted daily (Ae0‐24) and over the course of a week (Ae0‐168) during weeks 1 and 3 of dosing was measured.
Baseline Ae0‐24 was defined as the urine fluoride Ae0‐24 value on day 6 of period 1. To assess the completeness of urine collections for each participant, 24‐hour creatinine clearance was assessed using serum and urine creatinine concentrations on each day when urine collection was performed. No participants were identified as having an incomplete urine collection that would have impacted the analysis.
In period 1, blood samples for the determination of plasma fluoride concentrations were collected: at index time (time of dosing scheduled for period 2); at 4, 8, 12, and 24 hours later on days 1 and 6 of period 1; and before dosing and at 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 96, 120, and 168 hours after dosing on days 1 and 15 of period 2. Plasma PK parameters evaluated included area under the concentration‐time curve (AUC) from 0 to 24 hours after dosing (AUC0‐24), area under the concentration‐time curve from 0 to 168 hours after dosing (AUC0‐168), maximum plasma fluoride concentration, and time to maximum plasma fluoride concentration.
Analytical Methods
Fluoride concentrations in plasma and fluoride excretion in urine were analyzed by a commercial vendor, Quest Diagnostics (Chantilly, Virginia), using direct ion‐specific electrode potentiometry using an Orion Fluoride Electrode (Thermo Fisher Scientific, Waltham, Massachusetts) with a separate reference electrode. The lower limit of quantitation (LLOQ) of the assay was 1.05 μM.
Data Analysis
Fluoride Ae0‐24 and Ae0‐168 were determined by the product of the urine concentration and volume during the 24‐hour or 168‐hour collection interval, respectively.
Plasma PKs were calculated using PhoenixWinNonlin software version 7 (Certara, Princeton, New Jersey). Maximum plasma fluoride concentration and time to maximum plasma fluoride concentration values were obtained directly from plasma concentration–time data. AUC was calculated using the linear up/log down trapezoidal method. Fluoride concentration values that were below the LLOQ of the assay (1.05 μM) in plasma and urine were imputed as being half the LLOQ (ie, 0.526 μM).
Statistical calculations were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). To assess steady‐state urine fluoride, individual urine fluoride Ae0‐24 changes from baseline were evaluated in a linear mixed‐effects model. 9 The 95% confidence intervals (CIs) for the least‐squares means (LSM) by treatment were constructed. Similarly, the LSM differences (each of the 2 MK‐8507 dose levels vs placebo) and 90%CIs from this model were constructed.
The primary hypothesis of the study was that the true mean difference between MK‐8507 and placebo in the change from baseline of the daily amount of fluoride excreted in the urine Ae0‐24 averaged over 7 days following the administration of at least 1 dose level of MK‐8507 at steady state was ≤57 μmol. To assess and compare urine and plasma fluoride following single and multiple doses of MK‐8507, individual urine fluoride Ae0‐168 or plasma fluoride AUC0‐168 values were natural log (ln)‐transformed and evaluated with a linear mixed‐effects model. The 95%CIs for the LSMs by treatment and week were constructed on the ln‐scale. The LSM differences (week 3/week 1) and 90%CI of the LSM differences were back‐transformed to obtain the geometric mean accumulation ratio (week 3/week 1) and 90%CI of the geometric mean ratio.
Power
At the design stage, a between‐subject standard deviation (SD) estimate of 7 (at low dose, or 27 at high dose) for Ae0‐24 change from baseline was obtained using data from an earlier pilot trial of urinary fluoride excretion PK. 9 Assuming that the true between‐subject SD for change from baseline following multiple dosing was similar to this value, then with 6 subjects receiving MK‐8507 per panel and 6 subjects receiving placebo (pooled across 2 MK panels), a type I error rate of 0.05, and a 1‐sided test, there was 80% probability that the upper limit of the 2‐sided 90%CI for the true mean difference (MK‐8507–placebo) in change from baseline at period 2, week 3 would be ≤57 μmol, if the true mean difference was ≤49 (or ≤25 if SD = 27) μmol.
Safety Analysis
Safety was monitored throughout the trial by repeated clinical and laboratory evaluations.
Results
Participant Disposition and Baseline Characteristics
A total of 18 participants were enrolled in the trial; 9 participants were included in each cohort, with 6 participants randomized to MK‐8507 and 3 to placebo. All participants completed the trial per protocol. The trial was initiated on September 22, 2017, and completed on December 13, 2017. Baseline characteristics of participants are described in Table 1.
Table 1.
Demographic and Baseline Clinical Characteristics
| Characteristic | Placebo n = 6 | MK‐8507 200 mg n = 6 | MK‐8507 800 mg n = 6 |
|---|---|---|---|
| Age, y, mean (SD) | 36.8 (6.2) | 37.2 (13.2) | 38.5 (7.8) |
| Range | 25‐42 | 21‐52 | 29‐47 |
| Sex, n, % | |||
| Male | 6 (100.0) | 5 (83.3) | 4 (66.7) |
| Female | 0 (0.0) | 1 (16.7) | 2 (33.3) |
| Race, n % | |||
| Black or African American | 5 (83.3) | 2 (33.3) | 2 (33.3) |
| White | 0 (0.0) | 4 (66.7) | 2 (33.3) |
| Multiple | 1 (16.7) | 0 (0.0) | 2 (33.3) |
| Ethnicity, n, % | |||
| Hispanic or Latino | 0 (0.0) | 1 (16.7) | 2 (33.3) |
| Not Hispanic or Latino | 6 (100.0) | 5 (83.3) | 4 (66.7) |
| Weight, kg, mean (range) |
86.3 (69.4‐99.2) |
83.3 (61.3‐106.5) |
92.0 (82.4‐106.9) |
| Height, cm, mean (range) | 176 (166‐193) | 176 (163‐187) | 173 (162‐188) |
| BMI, kg/m2, mean (range) |
27.8 (24.0‐30.7) |
26.6 (23.1‐30.6) |
30.9 (29.1‐33.1) |
BMI, body mass index; SD, standard deviation.
Fluoride PK Parameters
Geometric mean Ae0‐24 for fluoride on period 1, day 1 (unrestricted fluoride exposure) was 58.4 μmol (SD, 21.0; geometric percent coefficient of variance, 33.3%; range, 36.4‐104; n = 17; 1 participant was not included in the analysis due to an incomplete urine collection), which decreased to 29.7 μmol (SD, 7.51; geometric percent coefficient of variance, 27.3%; range, 16.0‐43.4; n = 18) on day 6 (after 5 days on a fluoride‐restricted diet). Change from baseline in urine fluoride Ae0‐24 following administration of MK‐8507 or placebo for 3 weeks is shown in Table 2. Change from baseline in urine fluoride Ae0‐24 following administration of MK‐8507 demonstrated an increase in fluoride excretion in an approximately dose‐proportional manner. The amount of fluoride excreted in urine was higher after week 3 of MK‐8507 administration with 800‐mg dosing compared with week 1 (49% increase), whereas minimal change was observed with 200‐mg dosing (14% increase) (Table 3).
Table 2.
Change From Baseline in Urine Fluoride Ae0‐24 Averaged Over 7 Days in Healthy Participants Administered MK‐8507 (200 or 800 mg) or Placebo Once Weekly for 3 Weeks Under Conditions of a Fluoride‐Restricted Diet
| Amount of Fluoride Excreted in Urine, μmol | Placebo n = 6 | MK‐8507 200 mg n = 6 | MK‐8507 800 mg n = 6 |
|---|---|---|---|
| Baseline, mean (SD) a | 30.7 (8.4) | 31.3 (8.6) | 30.0 (6.8) |
| Poststudy drug, mean (SD) b | 24.5 (5.1) | 44.3 (4.3) | 98.5 (11.5) |
| Least‐squares mean change (95%CI) |
−6.3 (−12.8 to +0.3) |
+13.5 (+7.0 to +20.1) |
+68.8 (+62.3 to +75.4) |
| Difference in least‐squares mean vs placebo (90%CI) | … |
+19.8 (+12.2 to +27.4) |
+75.1 (+67.5 to +82.7) |
Ae0‐24, amount of fluoride excreted daily; CI, confidence interval; SD, standard deviation.
Defined as day 6 of period 1, following a fluoride‐restricted diet for 5 days.
Mean of Ae0‐24 recorded daily from day 15 to day 21 in period 2.
Table 3.
Urine Fluoride PK in Healthy Participants Administered 3 Weekly Doses of MK‐8507 (200 or 800 mg) Under Conditions of a Fluoride‐Restricted Diet
| Urine | ||||||||
|---|---|---|---|---|---|---|---|---|
| MK‐8507 200 mg | MK‐8507 800 mg | |||||||
| Week 1 | Week 3 | Week 1 | Week 3 | |||||
| Fluoride PK parameters | n | GM (95%CI)/[%CV] | n | GM (95%CI)/[%CV] | n | GM (95%CI)/[%CV] | n | GM (95%CI)/[%CV] |
| Ae0‐168 a, μmol | 5 | 270 (230‐317) | 6 | 309 (266‐358) | 5 | 459 (391‐539) | 5 | 682 (581‐800) |
|
MK‐8507 200 mg week 3/MK‐8507 200 mg week 1 |
MK‐8507 800 mg week 3/MK‐8507 800 mg week 1 |
|||||||
| GMR (90%CI) | GMR (90%CI) | |||||||
| Ae0‐168 a , μmol | 1.14 (0.99‐1.32) | 1.49 (1.27‐1.74) | ||||||
Ae0–168, amount excreted in urine during the 168‐hour period after dosing; CI, confidence interval; %CV, geometric coefficient of variation; GM, geometric mean; GMR, geometric least‐squares mean ratio; PK, pharmacokinetic.
Back‐transformed least‐squares mean and CI from linear mixed‐effects model performed on natural log‐transformed values.
Plasma fluoride levels in period 1 were largely below the LLOQ (day 1: 69/90 [77%]; day 6: 85/90 [94%]; 154/180 [86%] total samples), with no difference seen with visual inspection between days 1 (fluoride‐unrestricted diet) and day 6 (fluoride‐restricted diet). Plasma fluoride levels from period 2 were assessed; however, a large number of samples had fluoride concentrations below the LLOQ (430/468 [92%] samples). There was no clear distinction between the fluoride concentrations at either of the 2 dose levels of MK‐8507 or placebo and no clear distinction between values from any treatment group from week 1 to week 3. Due to the limitation of fluoride plasma detection, fluoride renal clearance values were not reported.
Safety
No drug‐related adverse events were reported by any participants.
Discussion
MK‐8507, a novel investigational nonnucleoside reverse transcriptase inhibitor that is being evaluated as a once‐weekly drug in combination with islatravir 20 mg in clinical trials for the treatment of people living with HIV‐1 (PLWH), may release fluoride through metabolism. Therefore, this clinical trial was conducted to characterize fluoride PK following MK‐8507 multiple‐dose administration.
Using data from an earlier pilot trial of urinary fluoride excretion PK, 9 an increase in fluoride intake of 1.89 mg/d (the difference between the maximum recommended intake of 4.8 mg and the mean US population intake of 2.91 mg) is expected to result in a mean increase of 57 μmol in urinary fluoride Ae0‐24 (averaged over a 7‐day dosing interval). Therefore, an increase from baseline of 57 μmol in urinary fluoride Ae0‐24 (averaged over 7 days and corrected for placebo) was defined as the upper limit for acceptable change in fluoride excretion at steady state for participants administered MK‐8507. Urine fluoride excretion data following multiple weekly administration of a 200‐mg dose of MK‐8507 resulted in a mean net increase of 19.8 μmol over placebo, whereas multiple weekly 800‐mg doses resulted in a 75.1‐μmol increase. Therefore, the primary hypothesis of this study (as defined in the Methods) was supported at the 200‐mg dose level, but not at the 800‐mg dose level.
Interpolating data between the 200‐ and 800‐mg doses, based on an assumed linear relationship between MK‐8507 dose and estimated mean daily fluoride released at steady state, suggests that a person consuming a diet containing an average US fluoride intake, and administered ≤400 mg MK‐8507 once weekly, would not exceed the RfD for fluoride designated by the US Environmental Protection Agency. However, an 800‐mg dose of MK‐8507, double the highest therapeutic dose under clinical investigation, is associated with an increase in fluoride exposure that may result in PLWH exceeding the fluoride RfD in the United States. 10
Understanding the effects of MK‐8507 on fluoride exposure is important because PLWH would be expected to require long‐term treatment. Long‐term treatment with pharmacologic agents that are associated with increased fluoride exposure, such as the antifungal voriconazole, has been associated with adverse events that are consistent with skeletal fluorosis. 6 , 11 , 12 , 13 Plasma levels of fluoride in patients administered voriconazole with fluoride‐related adverse events have been reported to be ≥7.5 μmol/L, whereas ≈85% of plasma measurements in this trial, from individuals administered MK‐8507, were below the LLOQ of approximately 1 μmol/L. 11 , 12 , 13 Therefore, plasma fluoride measurements were ≥7.5‐fold lower for individuals administered MK‐8507 compared with levels noted from voriconazole treatment where deleterious effects were observed. Since plasma fluoride levels were frequently below the LLOQ, the usefulness of the plasma data may be questionable due to limited sensitivity. These findings are consistent with a corresponding trial that indicated that urine sampling offers a more consistent and accurate method of assessing fluoride exposure in participants administered a fluoride‐restricted diet relative to plasma measurement. 9 Data from the current trial demonstrated readily detectable fluoride levels in urine samples, which increased in a dose‐dependent manner.
To decrease variability due to dietary fluoride intake, the trial incorporated a fluoride restricted diet run‐in phase of 5 days, which was determined to be sufficient based on previously collected data. 9 However, urine fluoride excretion continued to decline slightly in some participants receiving placebo in period 2 (Table S1), indicating the value of comparing outcomes with a within‐group baseline and a parallel group receiving placebo in this trial. Fluorosis is not known to be an adverse event that is commonly observed in PLWH, but the risk of bone‐related adverse events needs to be considered when investigating new antiretroviral therapies. 14 Bone health is of concern for PLWH because highly active antiretroviral therapies, especially regimens comprising tenofovir disoproxil fumarate or a protease inhibitor, can increase the risk of reduced bone mineral density and osteoporosis compared with people without HIV. 14 , 15 The increased risk of bone pathology among PLWH is believed to be related to immune changes and cellular function rather than fluoride metabolism. 14 , 15
Limitations
In this trial, participants without HIV were administered a fluoride‐restricted diet to control for dietary fluoride when assessing the impact of MK‐8507 on plasma and urine fluoride levels. The PK of fluoride following administration of MK‐8507 may differ in individuals on an unrestricted diet. Likewise, fluoride exposure differs across geographic regions; for period 1, day 1 value for background fluoride exposure from this small, localized trial population may not be readily generalizable to a larger, geographically dispersed population. For example, the difference between constitutive urine excretion on day 1 of this study and fluoride excretion after implementing a fluoride‐restricted diet was ≈29 μmol, which equates to an average daily fluoride exposure of ≈0.98 mg for each study participant recruited at the single study center. This value is substantially lower that the reported average fluoride intake of 2.91 mg/d for a person living in the United States. 7 Demographic differences between placebo and active groups and the small sample size (n = 6 per group; Table 1) may limit the universal application of these findings to a larger, more diverse population.
Values presented for daily fluoride exposure are also mean daily values over the course of a week and do not account for peak daily concentrations or the rapid clearance of fluoride. The clinical relevance of periodic, as opposed to persistent, fluoride exposure above recommended levels is unclear given the equilibrium between bone and plasma levels of fluoride, although data from preclinical studies suggest no difference in outcomes when exposed to steady state vs oscillating concentrations of fluoride. 16
Other factors may be involved that can impact fluoride release which were not explored. The precise metabolic pathway of fluoride release from MK‐8507 is unknown. As such, there is potential for extrinsic factors that may impact the fluoride exposure following administration of MK‐8507. However, the theoretical maximum cannot be exceeded.
Conclusions
Given the expected modest increase in fluoride exposure following administration of MK‐8507 at therapeutic doses under clinical investigation (100‐400 mg once weekly), fluoride levels are not expected to exceed a clinically relevant threshold in most individuals. However, MK‐8507 administered at anticipated supratherapeutic doses increased fluoride exposure, as evidenced by increases in fluoride excretion in the urine.
Conflicts of Interest
G.G., D.J.R., S.Z., A.S., P.L., S.A.S., and M.I. are/were employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey (MSD) when the trial was conducted. C.T. is an employee of Celerion, Lincoln, Nebraska, the contract research organization engaged by MSD to conduct the trial. Funding for this research was provided by MSD.
Data Sharing Statement
MSD data sharing policy, including restrictions, is available at http://engagezone.msd.com/dsdocumentation.php. Requests for access to the clinical trial data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Supporting information
Supplemental Information
Acknowledgments
The authors thank the participants and clinical research staff who participated in the trial. Medical writing and editorial assistance, under the direction of the authors, was provided by ApotheCom (London, UK) in accordance with Good Publication Practice guidelines. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey.
References
- 1. Ankrom W, Schurmann D, Jackson Rudd D, et al. Single doses of MK‐8507, a novel HIV‐1 NNRTI, reduced HIV viral load for at least a week. Oral O416. Presented at: HIV Glasgow; October 5–8, 2020. (virtual event).
- 2. Ankrom W, Schaeffer A, Panebianco D, et al. Safety, tolerability and pharmacokinetics following single‐ and multiple‐dose administration of the novel NNRTI MK‐8507 with a midazolam interaction arm. Poster presented at: HIV Glasgow; October 5–8, 2020. (virtual event). Poster P099.
- 3. Palmer CA, Gilbert JA. Position of the Academy of Nutrition and Dietetics: the impact of fluoride on health. J Acad Nutr Diet. 2012;112(9):1443‐1453. [DOI] [PubMed] [Google Scholar]
- 4. Martinez‐Mier EA. Fluoride: its metabolism, toxicity, and role in dental health. J Evid Based Complementary Altern Med. 2012;17(1):28‐32. [Google Scholar]
- 5. Aoun A, Darwiche F, Al Hayek S, Doumit J. The fluoride debate: the pros and cons of fluoridation. Prev Nutr Food Sci. 2018;23(3):171‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sidhu HS, Venkatanarasimha N, Bhatnagar G, Vardhanabhuti V, Fox BM, Suresh SP. Imaging features of therapeutic drug‐induced musculoskeletal abnormalities. Radiographics. 2012;32(1):105‐127. [DOI] [PubMed] [Google Scholar]
- 7. US Environmental Protection Agency, Health and Ecological Criteria Division, Office of Water . Fluoride: exposure and relative contribution analysis. https://www.epa.gov/sites/production/files/2019‐03/documents/fluoride‐exposure‐relative‐report.pdf. Accessed July 29, 2020.
- 8. Fawell J, Bailey K, Chilton J, Dahi E, Fewtrell L, Magara Y. Fluoride in Drinking Water. Published on behalf of the World Health Organization. London: IWA Publishing; 2006. [Google Scholar]
- 9. Gillespie G, Zhang S, Schaeffer A, Tomek C, Larson P, Aubrey Stoch A, Iwamoto M. A phase 1 trial to evaluate the relationship between fluoride intake and urinary fluoride excretion in healthy participants. J Clin Pharm. 2022;62(2):190‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. (SCHER) SCoHaER . Critical review of any new evidence on the hazard profile, health effects, and human exposure to fluoride and the fluoridating agents of drinking water. https://op.europa.eu/en/publication-detail/-/publication/3d3b8529-c2c2-4ac2-adb9-f232e936972e. Published 2010. Accessed September, 2021.
- 11. Wermers RA, Cooper K, Razonable RR, et al. Fluoride excess and periostitis in transplant patients receiving long‐term voriconazole therapy. Clin Infect Dis. 2011;52(5):604‐611. [DOI] [PubMed] [Google Scholar]
- 12. Gerber B, Guggenberger R, Fasler D, et al. Reversible skeletal disease and high fluoride serum levels in hematologic patients receiving voriconazole. Blood. 2012;120(12):2390‐2394. [DOI] [PubMed] [Google Scholar]
- 13. Moon WJ, Scheller EL, Suneja A, et al. Plasma fluoride level as a predictor of voriconazole‐induced periostitis in patients with skeletal pain. Clin Infect Dis. 2014;59(9):1237‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kruger MJ, Nell TA. Bone mineral density in people living with HIV: a narrative review of the literature. AIDS Res Ther. 2017;14(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ofotokun I. Deciphering how HIV‐1 weakens and cracks the bone. Proc Natl Acad Sci U S A. 2018;115(11):2551‐2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Catani DB, Tenuta LM, Andaló FA, Cury JA. Fluorosis in rats exposed to oscillating chronic fluoride doses. Braz Dent J. 2010;21(1):32‐37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information


