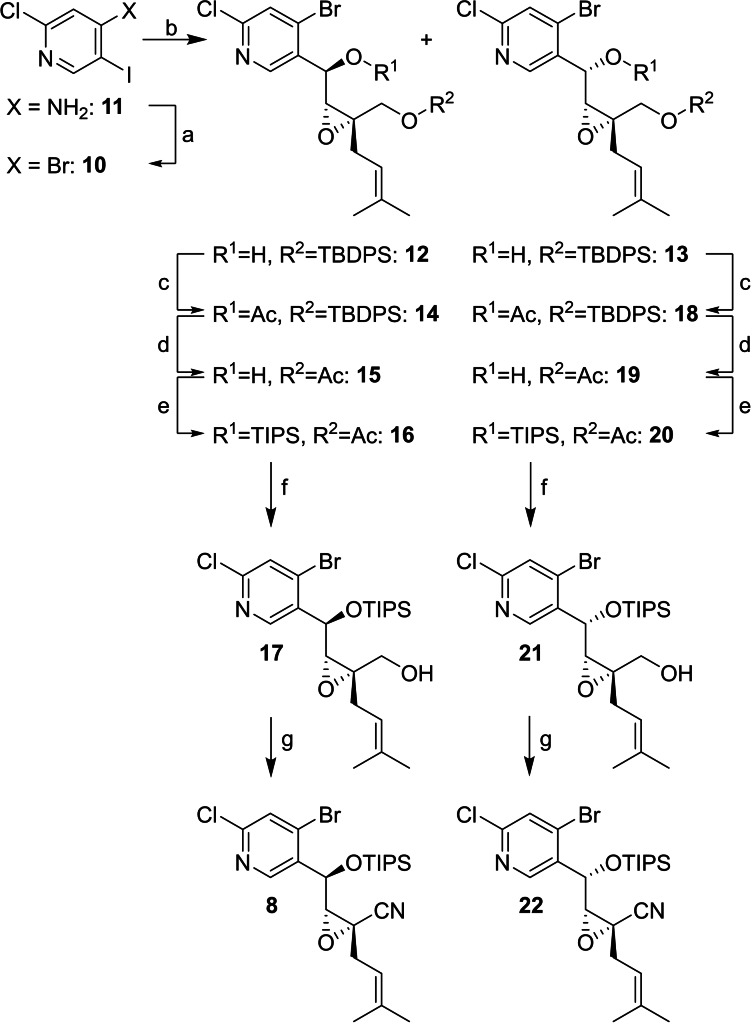
Scheme 2.
Synthesis of cyclization precursors 8 and 22: (a) t‐BuONO, CuBr2, MeCN, 0 °C to rt, 97 %; (b) i‐PrMgCl, THF, −40 °C, 45 min; 9, −40 °C, 1.5 h; 49 % for 12, 42 % for 13; (c) Ac2O, pyridine, DMAP (0.1 eq.), CH2Cl2; 97 % for 14, 98 % for 18; (d) TBAF, HOAc, THF; 86 % for 15, 90 % for 19; (e) TIPSOTf, 2,6‐lutidine, CH2Cl2; (f) LiOH, THF/H2O 5 : 1; 55 % over two steps for 17, 71 % over two steps for 21; (g) TEMPO (0.2 eq.), NH4OAc, BAIB, MeCN/H2O 9 : 1; 70 % for 8, 60 % for 22.