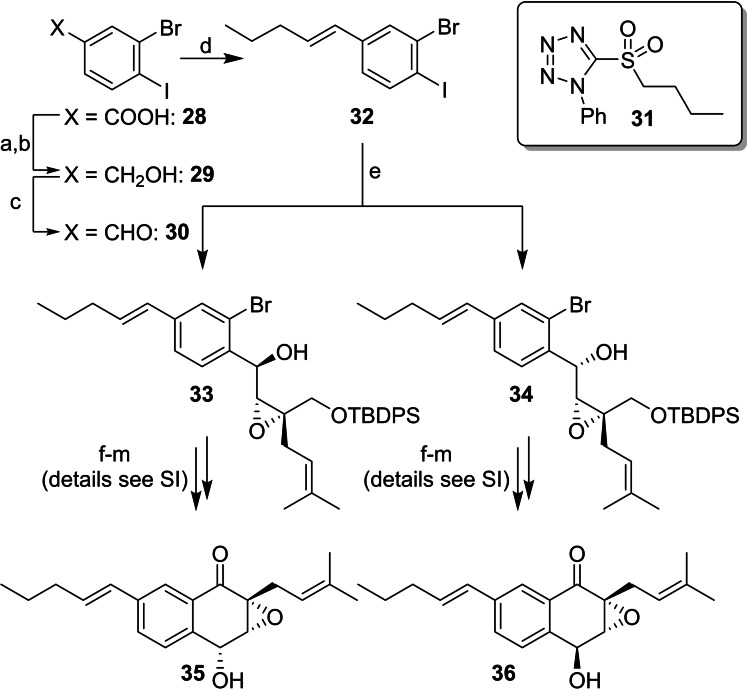
Scheme 4.
Total synthesis of C‐analogs 35, ent ‐35, 36, and ent ‐36 of floyocidin‐B, exemplarily shown for one diastereomeric series: (a) (COCl)2, DMF, CH2Cl2; (b) LiBH4, THF; 93 % over two steps; (c) (COCl)2, DMSO, CH2Cl2, −78 °C, 15 min; 29, −78 °C, 90 min; DIPEA, −78 °C to 0 °C, 30 min; quant. used as a crude; (d) 31, KHMDS, THF, −55 °C, 1 h 10 min; 32, −55 °C, 1 h; 75 % (E/Z 94 : 6); (e) i‐PrMgCl, THF, −40 °C, 45 min; 9, −40 °C, 1.5 h; 42 % for 33, 42 % for 34; (f) Ac2O, pyridine, DMAP (0.1 eq.), CH2Cl2; 97 %/99 %; (g) TBAF, HOAc, THF; (h) DBU, CH2Cl2; 87 %/79 % over two steps; (i) TIPSOTf, 2,6‐lutidine, CH2Cl2; (j) LiOH, THF/H2O 5 : 1; 46 %/63 % over two steps; (k) TEMPO (0.2 eq.), NH4OAc, BAIB, MeCN/H2O 9 : 1; 48 %/41 %; (l) n‐BuLi, THF, −100 °C, 20 min; 5 min without cooling; (m) TBAF, HOAc, THF; 58 % over two steps for 35, 31 % over two steps for 36.