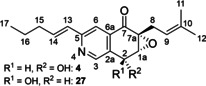
Table 1.
Comparison of NMR data and optical rotation of isolated and synthesized compounds 4 and 27.
|
| ||||||
|---|---|---|---|---|---|---|
|
|
Floyocidin‐B (isolated NP) |
4 (synthetic) |
27 (synthetic) |
|||
|
Position |
1H‐NMR |
13C‐NMR |
1H‐NMR |
13C‐NMR |
1H‐NMR |
13C‐NMR |
|
|
H (500 MHz)[a] |
C (125 MHz)[a] |
H (600 MHz)[a] |
C (150 MHz)[a] |
H (500 MHz)[a] |
C (125 MHz)[a] |
|
1a |
3.82, d (1.9) |
61.1 |
3.83, d (1.9) |
61.1 |
3.82, d (2.3) |
61.9 |
|
2 |
5.17, s, br |
65.3 |
5.18, s, br |
65.3 |
5.21, d (2.3) |
64.9 |
|
2a |
|
133.8 |
|
133.8 |
|
133.6 |
|
3 |
8.74, s |
150.0 |
8.75, s |
149.9 |
8.61, s |
152.5 |
|
5 |
|
157.5 |
|
157.5 |
|
158.5 |
|
6 |
7.67, s |
117.1 |
7.68, s |
117.1 |
7.72, s |
117.5 |
|
6a |
|
137.7 |
|
137.7 |
|
138.2 |
|
7 |
|
195.4 |
|
195.4 |
|
195.5 |
|
7a |
|
63.1 |
|
63.1 |
|
62.1 |
|
8 |
2.81, dd (15.2, 8.0) |
27.2 |
2.82, dd (15.2, 8.0) |
27.2 |
2.84, dd (15.2, 8.0) |
27.5 |
|
|
2.64, dd (15.2, 6.9) |
|
2.65, dd (15.2, 6.9) |
|
2.63, dd (15.2, 6.9) |
|
|
9 |
5.14, m |
117.8 |
5.14, m |
117.8 |
5.15, m |
117.8 |
|
10 |
|
137.0 |
|
137.0 |
|
137.0 |
|
11 |
1.69, s |
18.1 |
1.70, s |
18.1 |
1.69, s |
18.1 |
|
12 |
1.73, s |
26.0 |
1.74, s |
26.0 |
1.73, s |
26.0 |
|
13 |
6.55, dt (15.8, 1.5) |
130.1 |
6.56, dt (15.8, 1.5) |
130.0 |
6.56, dt (15.8, 1.5) |
130.1 |
|
14 |
6.78, dt (15.8, 7.1) |
138.5 |
6.79, dt (15.8, 7.1) |
138.5 |
6.82, dt (15.8, 7.1) |
139.0 |
|
15 |
2.27, m |
36.0 |
2.27, m |
36.0 |
2.27, m |
36.0 |
|
16 |
1.55, m |
23.2 |
1.56, m |
23.2 |
1.55, m |
23.2 |
|
17 |
0.98, t (7.4) |
14.1 |
0.99, t (7.4) |
14.1 |
0.98, t (7.4) |
14.1 |
|
7‐OH[b] |
– |
– |
|
– |
|
|
|
specific rotation |
= +160.0 (c 0.03; CHCl3) |
= +159.3 (c 0.27; CHCl3) |
= +179.3 (c 0.33; CHCl3) |
|||
|
specific rotation of ent‐series |
– |
=−206.0 (c 0.22; CHCl3) |
=−160.3 (c 1.20; CHCl3) |
|||
[a] MeOD (δ in ppm, multiplicity, J in Hz). [b] OH group not detectible in MeOD.