FIGURE 1.
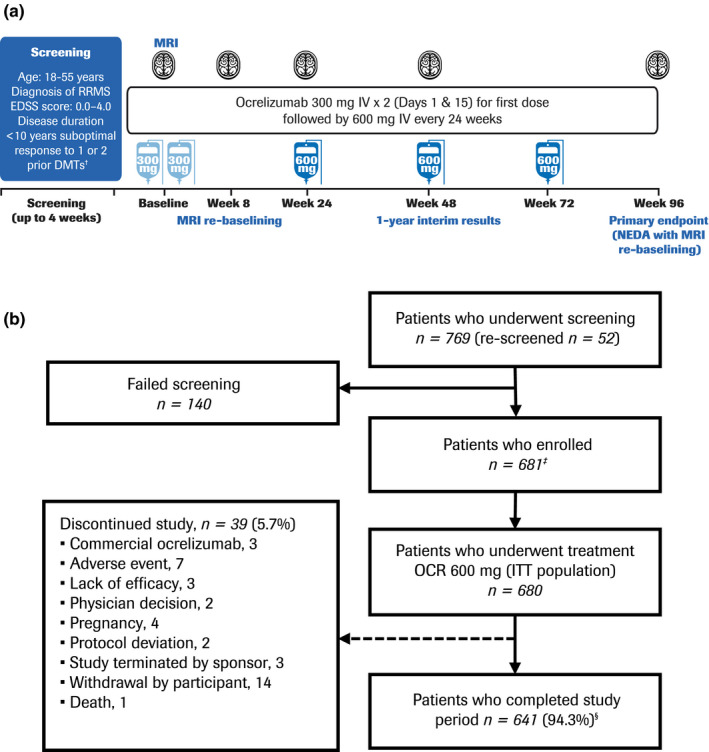
Study design (a) and patient disposition (b). †Patients were treated with a disease‐modifying therapy (DMT) for at least 6 months and with suboptimal efficacy within the last 12 months whilst on the DMT. ‡One patient discontinued before receiving treatment. §Of patients who completed the study treatment period, 154 discontinued for “other reasons” before safety follow‐up (of whom 152 took commercial ocrelizumab); 438 rolled over into the long‐term extension study (one additional patient also rolled over); 40 withdrew consent and did not enter safety follow‐up (of whom 25 took commercial ocrelizumab); eight patients entered safety follow‐up; and one patient discontinued before safety follow‐up by “physician decision” (this patient also took commercial ocrelizumab). Note: The per‐protocol (PP) population consisted of 560 patients. Reasons for exclusion from the PP population were: full course of ocrelizumab treatment not completed (n = 37); major protocol deviation deemed to potentially affect the efficacy endpoints (n = 56); and magnetic resonance imaging (MRI) performed after ocrelizumab infusion (n = 80). EDSS, Expanded Disability Status Scale; ITT, intention‐to‐treat; IV, intravenous; NEDA, no evidence of disease activity; OCR, ocrelizumab; RRMS, relapsing‐remitting multiple sclerosis
