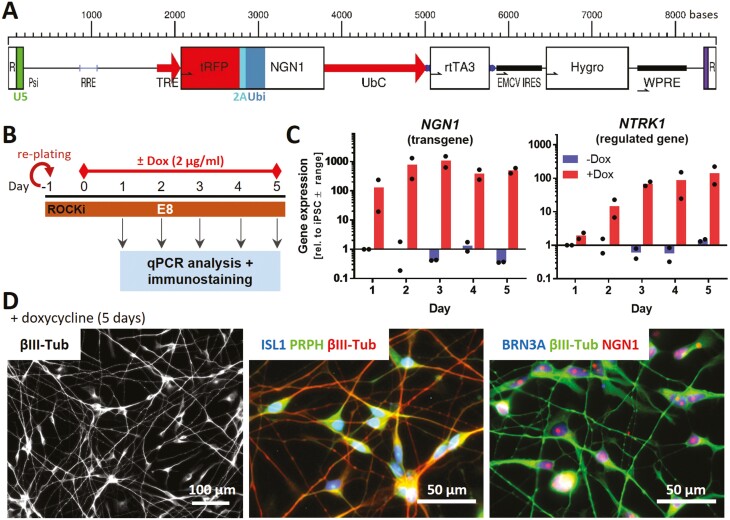
Figure 2.
Generation of human iPSCs with inducible expression of ectopic NGN1. (A) Structure of the lentiviral construct used to generate an NGN1-overexpressing iPSC line. NGN1 is expressed as fusion protein with turbo red fluorescent protein (tRFP). tRFP and NGN1 are linked via a 2A region and ubiquitin (Ubi) to ensure the precise cleavage of NGN1 inside cells. Gene expression is controlled via the tetracycline-on system (TRE promotor). This system uses a reverse tetracycline-controlled transactivator (rtTA3) driven by the UbC promoter. R, repeat region of the HIV long terminal repeat (LTR) region; U5 (green), U5ʹ region of the HIV LTR; Psi, packaging sequence; RRE, Rev response element; TRE, tetracycline response element; UbC, ubiquitin C promotor; EMCV IRES, encephalomyocarditis virus internal ribosomal entry site; Hygro, hygromycin resistance; WPRE, woodchuck hepatitis virus post-translational regulatory element. (B) Experimental setup to assess the NGN1 transgene expression. Cells were exposed for 5 days to doxycycline (0 or 2 µg/mL). (C) Gene expression of NGN1 and its downstream-regulated gene NTRK1 were monitored daily in control (-Dox) and doxycycline-exposed cells. Gene expression was quantified by RT-qPCR. Data are given relative to iPSC (control, day 1). Data displayed as bars are means of 2 biological replicates (dots). (D) Immunofluorescence images of cells treated with doxycycline for 5 days. Cells were labeled with antibodies against β-III tubulin (βIII-Tub), peripherin (PRPH) and the sensory neuronal transcription factors NGN1, ISL1, and BRN3A. Color code and scale bars are given in the images. More detail is given in Supplementary Fig. S2E,F.