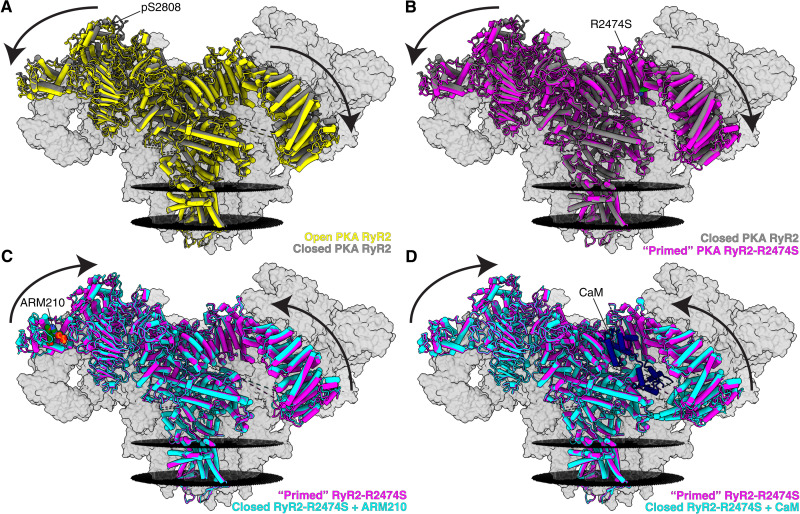
Fig. 1. Cryo-EM reconstructions of human RyR2 showing that the CPVT mutant RyR2-R2474S puts the channel into the primed state, and treatment with the Rycal ARM210 and CaM puts the channel back toward the closed state.
(A) Overlapped models of open PKA-phosphorylated RyR2 (PDB: 7U9R, yellow) and closed PKA-phosphorylated RyR2 (PDB: 7U9Q, gray). The arrows show that the cytosolic shell of the PKA-phosphorylated RyR2 shifts downward and outward when going from the closed to the open state. To facilitate visualization, only the front protomer is shown in colors, while the other three protomers are shown as gray transparent volumes. The positions of the sarcoplasmic reticular membranes are shown as black discs. Conditions include 10 mM ATP, 150 nM free Ca2+, and 500 μM xanthine. (B) Overlapped models of closed PKA-phosphorylated RyR2 (PDB: 7U9Q, gray) and primed PKA-phosphorylated RyR2-R2474S (PDB: 7U9X, magenta). The arrows show that the cytosolic shell of RyR2-R2474S shifts downward and outward compared to closed PKA-phosphorylated RyR2, similar to the structural changes observed for PKA-phosphorylated RyR2 going from the closed state to the open state. We define this intermediate between closed and open states as the primed state. (C) Overlapped models of primed PKA-phosphorylated RyR2-R2474S (PDB: 7U9X, magenta) and closed PKA-phosphorylated RyR2-R2474S + ARM210 (PDB: 7UA1, cyan). The arrows show that the cytosolic shell of PKA-phosphorylated RyR2-R2474S + ARM210 cytosolic domain shifts upward and inward compared to the RyR2-R2474S reversing the primed state back toward the closed state. (D) Overlapped models of primed PKA-phosphorylated RyR2-R2474S (PDB: 7U9X, magenta) and closed PKA-phosphorylated RyR2-R2474S + CaM (PDB: 7UA3, cyan). Similar to the effects of the Rycal ARM210, CaM reverses the primed state back toward the closed state.