Figure 1.
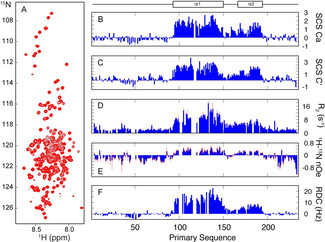
Characterisation of the structural and dynamic properties of CAHS‐8 in solution. A) The 15N‐1H correlation spectrum is characteristic of an intrinsically disordered protein. B, C) Secondary 13Ca and 13C′ chemical shifts showing strong α‐helical propensities from 95–195, divided into two distinct helices, α1 (E95‐F150) and α2 (V167‐L194). 15N relaxation (D; 850 MHz, E; 600 MHz), 15N‐1H RDCs (F), and PREs (see Figure S1) suggest the protein acts as a disordered chain comprising two highly populated α‐helices (303 K) and no persistent tertiary structure. The NMR spectrum was recorded at 350 μm (9.3 mg mL−1). The NMR assignments are deposited in BMRB (accession code 51115).
