Figure 4.
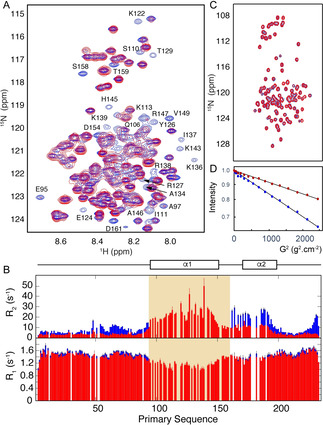
CAHS‐8 disordered domains remain flexible in gels that confine soluble proteins. A) Comparison of the 15N‐1H correlation spectra (293 K, 850 MHz) of CAHS‐8 in monomeric (blue) and gel (red) forms. Residues in helix α1 that disappear in the gel spectrum are annotated. B) Transverse (R2) and longitudinal (R1) 15N relaxation (293 K, 850 MHz) of monomeric (red) and gel (blue) CAHS‐8. Signals from the shaded region were not visible in the spectrum. C) 15N‐1H correlation spectra of the disordered domain of transcription factor ANP32a in buffer (blue) and gel (red) reveal that conformational sampling of ANP32a is unperturbed in gel. D) DOSY measurements of ANP32a in buffer (blue) and gel (red) reveal that translational diffusion of ANP32a is significantly reduced (4.8 compared to 9.6 10−11 m2s−1) in the gel.
