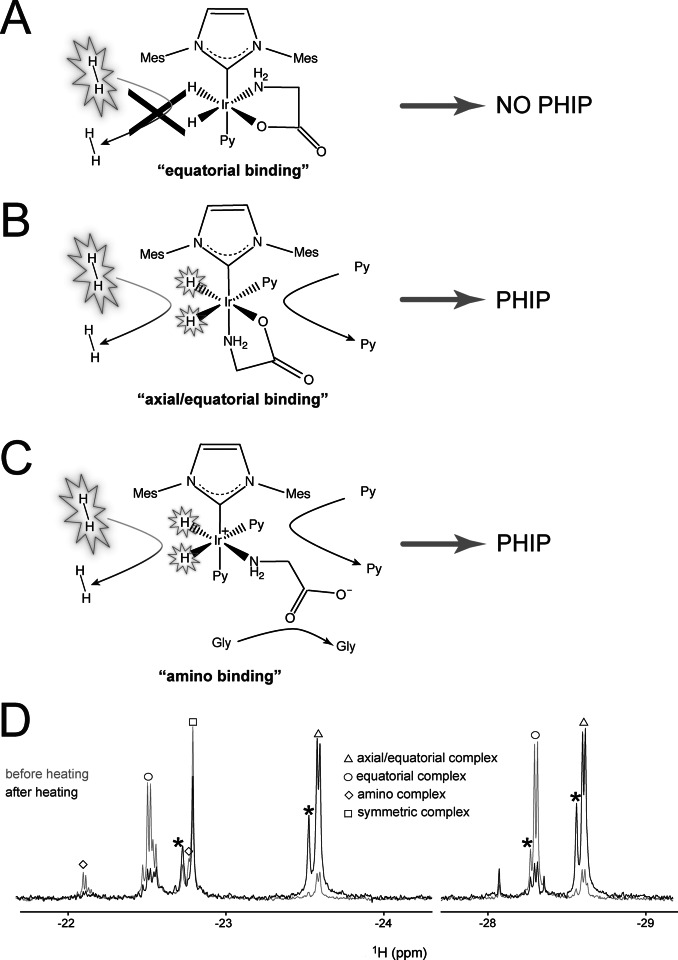
Figure 2.
A–C) Schematic representation of the main conformation geometries of glycine upon binding the Ir‐IMes complex in the presence of an excess of the co‐substrate pyridine. A) “Equatorial” binding. No ligand dissociation occurs on the NMR time scale, and accordingly p‐H2 refreshment cannot take place, abolishing nhPHIP for this complex. B) “Axial/equatorial” binding. Fast pyridine exchange is allowed and, consequently, reversible binding of p‐H2 and nhPHIP on the hydrides. C) “Amino” binding. Fast ligands exchange and, consequently, reversible binding of p‐H2 allows nhPHIP on the hydrides. Please note that a bidentate binding of the amino acid involving also the carboxy group in the axial position is not excluded (see Supporting Information). D) 1D NMR thermal spectra of the hydrides acquired in a 95 vol % [D4]MeOH, 5 vol % H2O solution of 0.66 mM glycine in the presence of 0.82 mM Ir‐IMes catalyst, 15.2 mM pyridine and 5 bar H2 at 278 K. The spectra were recorded at 500 MHz 1H resonance frequency, before and after sample heating, as described in the main text. A tentative assignment of the hydrides signals corresponding to the complexes in solution is indicated (see Supporting Information). Note that the structure observed for the signals at −22.15 and −22.5 results from the H/D isotope effect due to the amino group in the equatorial plane of the “amino” and “equatorial” complexes assigned to these signals, respectively. The signals marked with an asterisk originate from deuteration of one of the two hydrides in the complex.