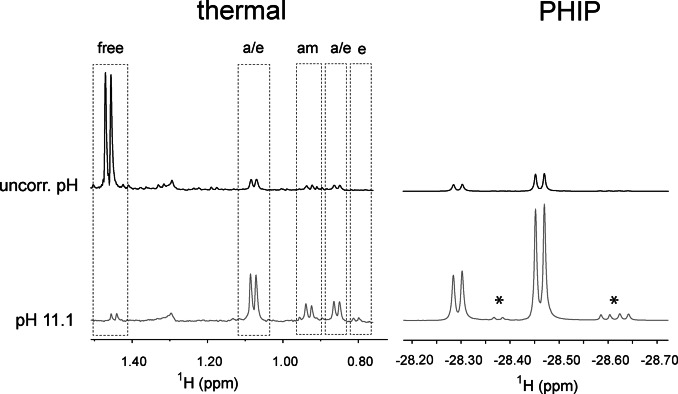
Figure 3.
Effect of pH on the association of alanine to the iridium complex (left) and on the nhPHIP‐NMR signals of the corresponding hydrides (right). The spectra at the top were acquired without pH correction, while the spectra at the bottom were measured in the presence of a 20 mM buffer of piperidine/piperidinium at pH 11.1. Left: NMR signals of the methyl protons of alanine after catalyst activation at 50 °C for 7.5 minutes. The signal assignment for the amino acid complexes is indicated as “a/e” (axial/equatorial), “am” (amino) and “e” (equatorial). Right: nhPHIP‐NMR signals of the high‐field hydrides for the diastereomeric “axial/equatorial” complexes formed by alanine. The signals marked with asterisks originate from impurities. The spectra were acquired under identical conditions (100 μM 13C Ala, 0.83 mM Ir‐IMes catalyst, 15 mM pyridine, 5 bar 51 %‐enriched p‐H2 and 0.5 vol‐% water) at 278 K.