Abstract
Inflammation seems to play a critical role in the development and progression of different cancers. Neutrophil‐to‐lymphocyte ratio (NLR) is an easily measurable marker of systemic inflammation. The purpose of this systematic review and meta‐analysis was to evaluate the prognostic role of the pre‐treatment NLR, in terms of overall survival (OS) and disease‐free survival (DFS), in patients with primary head and neck squamous cell carcinoma (HNSCC) treated by surgery alone or followed by chemo/radiotherapy. This systematic review was performed according to the guidelines reported in the Cochrane Handbook and the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement. Meta‐analysis of OS and DFS was performed using the inverse of variance test. Fixed‐effect models were used on the basis of the presence of heterogeneity. Risk of bias assessment and trial sequential analysis (TSA) were also performed; the quality of the evidence was evaluated via the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. The analysis revealed that a higher value of pre‐treatment NLR correlates with a statistically significant decrease of OS (HR, 1.56; 95% CI: [1.35, 1.80]; p < 0.00001) and a lower DFS (HR, 1.64; 95% CI: [1.30, 2.07]; p < 0.0001) in HNSCC patients.
Keywords: cancer prognosis, head and neck squamous cell carcinoma, neutrophil to lymphocyte ratio, NLR
1. INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world, with 890 000 new cases and 450 000 deaths in 2018 worldwide. 1 Despite improvements in research and therapy made in the last decades, survival has not significantly improved and the 5 years overall survival (OS) rate is still less than 50%. 2 Classic prognostic factors are not sufficient to predict patients’ prognosis, due to the heterogeneity of molecular mechanisms and tumour behaviours related to HNSCC. For these reasons, there has been an intensified interest in biomarkers’ discovery for early diagnosis, prognosis and personalized treatment.
In this scenario, inflammatory biomarkers became a reliable and accessible source of information to investigate and correlate to clinical outcomes. 3 Evidence suggests that inflammation contributes to tumour development and metastasis. A high number of neutrophils and macrophages infiltrating the tumour microenvironment seems to be associated with worse outcomes 4 , 5
The most commonly reported inflammatory parameters are C‐reactive protein, neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) and lymphocyte‐to‐monocyte ratio (LMR). 3 Among these, NLR represents the most studied and promising clinical biomarker. 6 Neutrophils might help carcinogenesis in different ways, as the release of prostaglandin E2 and proteases, the destruction of the extracellular matrix, inhibition of T‐cells and the modulation of macrophage activity, facilitating tumour growth and its progression. 7
The purpose of this systematic review and meta‐analysis was to evaluate the prognostic role of the pre‐treatment NLR, in terms of OS and disease‐free survival (DFS), in patients with primary HNSCC treated using surgery followed or not by adjuvant therapies. Additionally, Trial Sequential Analysis (TSA) for the time‐to‐event outcomes was performed aiming to investigate the statistical power of the reported meta‐analytic findings.
2. MATERIALS AND METHODS
2.1. Protocol
This systematic review was performed according to the Cochrane Handbook, 8 the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 9 and recommendations from the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) 10 group. The protocol was designed a priori and registered on the online database PROSPERO (CRD42020216751).
2.2. Search strategy
The literature search was performed in the PubMed, Web of Science and Embase databases up to December 2020. The following search string has been used on PubMed: (HNSCC OR head and neck squamous cell OR oral OR larynx OR pharynx OR tongue OR oropharynx OR hypopharynx OR buccal OR mouth OR SCCHN OR OSCC OR oral cancer) AND (NLR OR neutrophil lymphocyte ratio OR neutrophil‐to‐lymphocyte ratio OR neutrophil‐lymphocyte ratio OR Systemic inflammatory markers OR hematologic markers OR neutrophil‐to‐lymphocyte ratio). The string was modified to adapt it for each search engine used. Moreover, bibliographies of systematic reviews and grey literature were hand‐revised to gather additional studies.
2.3. Eligibility criteria
To be included, studies had to fulfil the following criteria: (i) prospective and retrospective cohort studies analysing prognostic impact of peripheral blood NLR in HNSCC; (ii) patients with histologically confirmed diagnosis of primary HNSCC undergoing surgery as first treatment with or without adjuvant therapy; (iii) studies which evaluated the association between the pre‐treatment NLR values and survival outcomes, calculating at least one of the following parameters: OS and Disease Free Survival (DFS) (including the articles evaluating Recurrence Free Survival (RFS), which fell under the definition of DFS); (iv) a minimum number of 30 patients; (v) studies which used a single cut‐off value of the NLR to stratify patients; (vi); studies which directly reported either hazard ratio (HR) with its 95% Confidence Interval (CI) or the Kaplan–Meier graph (in this case, the HR of survival analysis and its 95% CI were estimated applying the method by Tierney et al. 11 ) and (vii) full‐text articles published in English.
The exclusion criteria were: (i) reviews, letters, or case reports; (ii) animal studies; (iii) studies with patients affected by HNSCC HPV+; (iv) patients with concurrent tumours.
2.4. Study selection, data collection and data items
The process of study selection was divided into multiple steps. First, authors screened for articles by reading only title and abstract. Full texts of publications, meeting the initial inclusion criteria, were analysed in the second round. At the end of the second phase, two reviewers (PM and DR) provided independently a final judgement of inclusion for the selected articles and notified such recommendations to a third author (GT). This author calculated a value of k‐statistic to ascertain the level of reviewers’ agreement. In cases of disagreement, the same author (GT) took a final decision. At the end of the selection process, papers fulfilling all inclusion criteria were included.
Data extraction was performed independently by two authors (PM and MM) using a specific extraction sheet; subsequently, data were double‐checked in a joint session with a third author (GT). The following parameters were extracted from each included study: name of the first author, year of publication, nation where the study was performed, type of study, age (mean or median) of cohort, head and neck tumour sub‐localization, staging, treatment, cut‐off methods, cut‐off values, outcomes, HRs and 95% CI for OS and DFS.
2.5. Quality of evidence and risk of bias assessment
The risk of bias (ROB) of the included studies was performed using parameters derived from the Reporting Recommendations for Tumour Marker Prognostic Studies (REMARK). 12 , 13 The scale consists of six parameters evaluating: samples, clinical data of the cohort, marker quantification, prognosis, statistics and classical prognostic factors. Based on the REMARK guidelines each factor was considered: adequate (A), inadequate (I) or not evaluable. Furthermore, an analysis of the ROB across studies was performed using Q and I2 tests.
The quality of the evidence was evaluated via the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) for each comparison between the study groups at the outcome level. 14 The evaluation was performed utilizing the GRADEpro platform (McMaster University, Hamilton, Canada ‐ https://gradepro.org).
2.6. Summary measures and planned methods for statistical analyses
2.6.1. Meta‐analysis
The pooled analyses were performed using the software Review Manager version 5.2.8 (Cochrane Collaboration, Copenhagen, Denmark; 2014). Only studies reporting HR and CI for the multivariate analysis were included. For survival analysis, the impact of NLR on OS and DFS, the natural logarithmic of the HR and its standard error (SE) were calculated using the Calculator function of Review Manager. Heterogeneity among studies was evaluated though Higgins Index (I2 ) and the chi‐square test and classified as follows: low heterogeneity (<30%), medium heterogeneity (30%–60%) and high heterogeneity (>60%).
Overall effects were compared using the inverse of variance test, setting a threshold of significance of p < 0.05. Only data assessed with multivariate analysis in the included studies were included in the meta‐analysis.
2.6.2. Trial sequential analysis (TSA)
TSA was carried out to assess the power of the meta‐analytic findings and adjust results to avoid type I and II errors. Data were analysed using metacumbounds command 15 in statistical Stata Statistical Software version 13.0 (StataCorp, College Station, TX). We used the O'Brien–Fleming spending function to calculate the monitoring boundaries because of its conservative behaviour. 16 Each cumulative z‐value was determined by a random‐effect model because the meta‐analysis showed high heterogeneity (I2 >60%). 17 The calculation of the information size is based on an a priori anticipated intervention effect (a priori information size, APIS), and setting a 20% relative risk reduction (RRR), 5% type I error and 20% type II error.
3. RESULTS
3.1. Study selection process and study features
A total of 3071 records were retrieved by the initial search. After the first screening process, 99 articles meet the inclusion criteria and were full‐text assessed. Subsequently, only 27 articles 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 were considered eligible (Table 1). The flowchart with details of the study selection process and reasons for exclusion is shown in Figure 1 and Supplementary materials (Table S1). Agreement between reviewers was excellent with a Cohen's kappa coefficient of 0.905.
TABLE 1.
Characteristics of studies included in this systematic review and meta‐analysis
| Study | Year | Country | Study design | Sample size | Age (mean) | Tumour site | Tumour stage | Treatment | NLR (mean) | Cut‐off values | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage I + II | Stage III + IV | OS | DFS/DSS/PFS | ||||||||||
| Bobdey et al. 18 | 2016 | India | Retrospective | 471 | 50(25–85) | OC | 124 | 347 | S | NA | 2.38 | NA | OS |
| Chen et al. 34 | 2020 | China | Retrospective | 473 | 63.48 ± 10,21 | L | 326 | 147 | S | 2.20 ± 1.45 | 1.96 | 1.96 | RFS |
| Chen’ et al. 36 | 2018 | China | Retrospective | 361 | 60 (35–87) median | L | 232 | 129 | S/S+A | NA | 2.45 | 2.45 | OS, PFS |
| Chen* et al. 35 | 2016 | China | Retrospective | 306 | 55 (17–87) median | OC | 243 | 63 | S | NA | 2.7 | 2.7 | OS, DFS |
| Chen° et al. 37 | 2018 | China | Retrospective | 708 | NA | OC | 335 | 373 | S/S+A | NA | 2.03 | NA | OS |
| de Almeida et al. 33 | 2019 | Canada | Retrospective | 551 | 61 ± 13,7 | OC | 355 | 195 | S/S+A | 3.1 ± 1.9 | 2.9 | 2.9 | OS, RFS |
| Fang et al. 28 | 2013 | Taiwan/ China | Retrospective | 226 | 52.47 (27–84) | OC | 91 | 135 | S/S+A | 2.88±2.05 | 2.44 | 2.44 | OS, DFS |
| Fu et al. 26 | 2016 | China | Retrospective | 420 | 60 (33–84) median | L | 0 | 420 | S | NA | 2.59 | 2.59 | OS, CSS |
| Hasegawa et al. 30 | 2020 | Japan | Retrospective | 433 | 63.3 ± 13.5 | OC | 301 | 132 | S | 2.5 ± 1.73 | 2.22 | 2.22 | OS, DSS |
| Ikeguchi 25 | 2016 | Japan | Retrospective | 59 | 68.7 ± 9.5 | NA | 0 | 59 | S/S+A | 3.6 | 5 | NA | OS |
| Kao et al. 22 | 2018 | Taiwan | Retrospective | 613 | 53.0 ± 11.38 | OC | 251 | 362 | S/S+A | 2.7 ± (1.78) | 2.28 | NA | OS |
| Lee et al. 19 | 2020 | Rep. of Korea | Retrospective | 291 | 63(24–91) median | OC | 163 | 128 | S/S+A | 2.61 ± 2.52 | 2.23 | 2.16 | OS, DFS |
| Lo et al. 32 | 2017 | Taiwan | Retrospective | 105 | 55.5 ± 11.5 | HP | 0 | 105 | S+A | NA | 3.22 | 3.22 | OS, DFS, DSS |
| Lu et al. 39 | 2020 | China | Retrospective | 120 | 55 (22–86) median | OC | 29 | 91 | S/S+A | NA | 2.8 | 2.8 | OS, DFS |
| Lu’ et al. 38 (training) | 2020 | China | Retrospective | 202 | NA | OC | 17 | 185 | S+A | NA | NA | NA | DFS |
| Lu’ et al. 38 (validation) | 2020 | Taiwan | Retrospective | 144 | NA | OC | 18 | 126 | S+A | NA | 2.6 | 2.6 | DFS |
| Song et al. 27 | 2014 | China | Retrospective | 146 | 57.5 (34–89) | HP | 56 | 90 | S/S+A | 2.68 (0.71–8.75) | 2.3 | 2.3 | OS |
| Szilasi et al. 41 | 2020 | Hungary | Retrospective | 156 | 58.1± 8.7 | OC, OP, HP, L | 50 | 106 | S/S+A | 2.8 | 3.9 | 3.9 | OS |
| Tazeen et al. 43 | 2020 | India | Retrospective | 112 | 47.03 (M)–55.88 (F) | OC | 44 | 68 | S/S+A | NA | 3.1 | 3.1 | OS, DFS |
| Tu et al. 29 | 2015 | China | Retrospective | 141 | 59 (36–87) median | L | 80 | 61 | S | NA | 2.17 | 2.17 | OS, DFS |
| Wang et al. 31 | 2016 | China | Retrospective | 120 | 60.0 ± 86 | L | 60 | 60 | S/S+A | NA | 2.79 | 2.79 | OS, RFS |
| Wu et al. 24 | 2017 | Taiwan | Retrospective | 262 | 51 median | OC | 262 | 0 | S/S+A | 2.18 (0.6–7) | 2.95 | 2.95 | OS, DFS, DSS |
| Xun et al. 20 | 2019 | China | Retrospective | 151 | 65 (44–84) median | L | 119 | 32 | S | NA | 2.2 | 2.2 | OS, PFS |
| Yang et al. 23 | 2018 | China | Retrospective | 197 | NA | HP | 30 | 167 | S/S+A | NA | 2.69 | 2.69 | OS, DFS, CSS |
| Ye et al. 42 | 2020 | China | Retrospective | 140 | 62,15 (median) | HP, L, OP | 84 | 56 | S/S+A | NA | NA | 2.77 | RFS, CSS |
| Zhang et al. 7 | 2019 | China | Retrospective | 103 | <40 and >60 | OC | 83 | 20 | S/S+A | 2.56 | 2.56 | 2.56 | DSS, RFS |
| Zhong et al. 44 | 2019 | China | Retrospective | 147 | 61.33 ± 13.31 | SN | 82 | 65 | S | 4.19 ± 1.33 | 4.25 | 4.25 | OS, DFS, DSS |
| Zhou et al. 40 | 2020 | China | Retrospective | 367 | 60.33 ± 11.84 | OC, OP, HP, L, ES, MS, Lp | 208 | 159 | S/S+A | NA | 2.81 | 2.81 | OS, DFS |
Abbreviations: A, adjuvant therapy; CSS, cancer‐specific survival; DFS, disease‐free survival; DSS, disease‐specific survival; ES, ethmoid sinus; HP, hypopharynx; L, larynx; LCR, loco‐regional recurrence; Lp, Lip; MS, maxillary sinus; NA, not reported; NLR, neutrophil lymphocyte ratio; OC, oral cavity; OP, oropharynx; OS: overall survival; PFS, progression‐free survival; S, surgery; SN, sinonasal.
FIGURE 1.
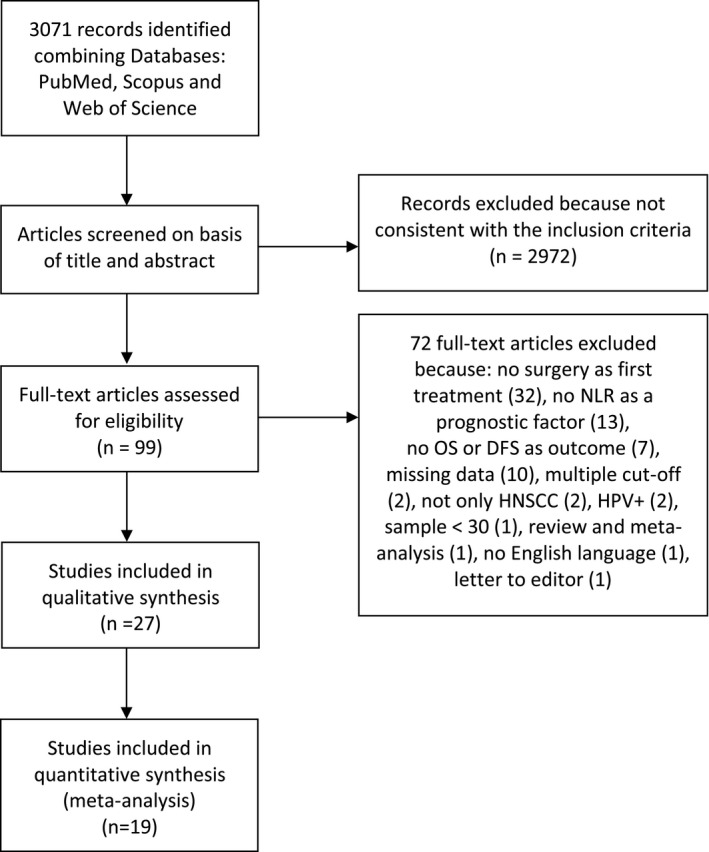
PRISMA flowchart of the selection process
All the selected studies were included in the qualitative analysis, consisting of 28 cohorts (Lu et al. 39 evaluated training and validation cohorts) with a total of 7525 patients. Of these, only 19 18 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 32 , 36 , 37 , 39 , 40 , 42 , 43 , 44 were included in the quantitative synthesis with a total of 4881 patients (Table 2). All the included studies were published between 2013 and 2020 with sample sizes ranging from 59 to 708. Twenty‐five studies 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 42 , 43 , 44 were conducted in Asia, one 33 in North America and one 41 in Europe (Table 1). Twenty‐three 18 , 19 , 20 , 21 , 22 , 23 , 24 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 studies included patients with TNM stages I‐II and III‐IV, three studies 25 , 26 , 32 included only patients in the stages III‐IV and one study 24 included exclusively patients in stages I‐II.
TABLE 2.
Synthesis of data extracted from the included studies related to outcomes pooled in the systematic review and meta‐analysis
| Study | Type of analysis | Follow‐up (month) | Overall Survival | Disease free survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (range) | Univariate | Log‐Rank | Multivariate | Univariate | Log‐Rank | Multivariate | ||||||||||
| HR | 95% C.I | p‐value | p‐value | HR | 95% C.I | p‐value | HR | 95% C.I | p‐value | p‐value | HR | 95% C.I | p‐value | |||
| Bobday et al. 18 | U+M | 22(0–98) median | 1.676 | 1.271–2.209 | 0 | 0.001 | 1.392 | 1.045–1.855 | 0.024 | |||||||
| Chen et al. 34 | U | NA | 2.79 | Estimated‡ | 0 | NA | NA | NA | ||||||||
| Chen’ et al. 36 | U+M | 47 (4–98) median | 2.53 | 1.66–3.84 | < 0.001 | <0.001 | 1.64 | 1.06–2.54 | 0.026 | |||||||
| Chen* et al. 35 | U | 34(6–60) median | NA | NA | NA | 0.267 | NA | NA | NA | 0.237 | ||||||
| Chen° et al. 37 | U+M | 43.28 median | 1.58 | 1.16–2.15 | 1.39 | 1.01–1.9 | ||||||||||
| de Almeida et al. 33 | U | NA | 1.17 | 1.09–1.26 | <0.001 | 1.08 | 1.01–1.16 | 0.03 | ||||||||
| Fang et al. 28 | U+M | NA | 2.04 | 1.036–4.014 | 0.034 | 0.034 | 1.181 | 1.046–1.333 | 0.007 | 1.72 | 1.038–2.849 | 0.031 | 0.031 | 1.126 | 1.013–1.252 | 0.028 |
| Fu et al. 26 | U+M | NA | 1.32 | 1.02–1.71 | 0.032 | 0.032 | 1.31 | 1.00–1.71 | 0.046 | |||||||
| Hasegawa et al. 30 | U+M | 59.1 (1–179) | NA | NA | NA | <0.001 | 2.3 | 1.42–3.72 | <0.001 | |||||||
| Ikeguchi 25 | U+M | 38.5 (5–108) | NA | NA | NA | 0.001 | 5.586 | 1.169–2.682 | 0.031 | |||||||
| Kao et al. 22 | U | NA | 1.759 | 1.320–2.345 | 0.0001 | <0.001 | ||||||||||
| Lee et al. 19 | U+M | 41 (3–144) | 2.01 | 1.15–3.53 | 0.015 | 0.001 | 1.78 | 1.01–3.14 | 0.045 | 2.1 | 1.39–3.15 | <0.001 | <0.001 | 1.82 | 1.12–2.94 | 0.015 |
| Lo et al. 32 | U+M | 50.0 (4–140) | 2.29 | 1.37–3.84 | 0.001 | 0.001 | 2.53 | 1.48–4.30 | 0.001 | 2.19 | 1.26–3.81 | 0.004 | 0.004 | 2.18 | 1.24–3.83 | 0.007 |
| Lu et al. 39 | U | 37.5 (3–92) median | 3.264 | 1.565–6.807 | 0.002 | NA | NA | NA | 0.165 | 2.417 | 1.195–4.891 | 0.014 | NA | NA | NA | 0.595 |
| Lu et al. 38 (training) | NA | 39.3 (4,8–79,8) | 1.612 | 0.807–3.22 | 0.176 | NA | NA | NA | ||||||||
| Lu et al. 38 (validation) | U+M | 39.3 (5,9–95,1) | 1.87 | 1.088–3.215 | 0.024 | 2.462 | 1.325–4.574 | 0.004 | ||||||||
| Song et al. 27 | U+M | NA | 2.79 | Estimated‡ | <0.001 | 2.36 | 1.33–4.18 | 0.003 | ||||||||
| Szilasi et al. 41 | U+M | 36(4,9–161) median | 2.1 | Estimated‡ | 0.001 | |||||||||||
| Tazeen et al. 43 | U+M | (6–29) | 1.91 | Estimated‡ | 0.002 | 1.171 | 0.449–3.053 | 0.747 | ||||||||
| Tu et al. 29 | U+M | 51 (5–102) median | 2.25 | Estimated‡ | 0.003 | 2.177 | 1.208–3.924 | 0.01 | 1.85 | Estimated‡ | 0.021 | 1.869 | 1.078–3.234 | 0.026 | ||
| Wang et al. 31 | U | NA | 1.994 | 1.089–3.649 | 0.25 | 0.02 | 1.921 | 1.107–3.335 | 0.02 | 0.017 | ||||||
| Wu et al. 24 | U+M | 67.1 (2–137) | 2.53 | Estimated‡ | <0.001 | 2.292 | 1.326–3.962 | 0.003 | 1.91 | Estimated‡ | 0.004 | 1.914 | 1.02–3.595 | 0.043 | ||
| Xun et al. 20 | U+M | NA | 0.22 | 0.12–0.41 | <0.001 | <0.001 | 3.02 | 1.28–7.10 | 0.011 | |||||||
| Yang et al. 23 | U+M | 30.95 (1–82) median | 1.49 | 1.02–2.18 | 0.04 | 0.033 | 0.95 | 0.63–1.43 | 0.796 | 1.6 | 1.11–2.31 | 0.012 | 0.012 | 0.83 | 0.56–1.24 | 0.363 |
| Ye et al. 42 | U+M | 51.7±0.34 | 1.1 | 1.0–1.1 | 0.001 | 0.004 | 2.16 | 1.30 – 3.5 | 0.003 | |||||||
| Zhang et al. 21 | U+M | 89.9 (7–205) | NA | NA | 0.643 | |||||||||||
| Zhong et al. 44 | U+M | 38.44 ± 14.69(OS); 38.13 ± 14.04 (DFS); 37.47 ± 13.15 (DSS) | 1.888 | 1.336–3.342 | <0.001 | 0.001 | 1.579 | 1.217–3.092 | 0.002 | 1.763 | 1.156–3.149 | <0.001 | 0.002 | 1.688 | 1.162–3.363 | <0.001 |
| Zhou et al. 40 | U+M | 27.2 (2–48) median | 2.151 | 1.363–3.394 | 0.001 | NA | 0.692 | 0.329–1.456 | 0.332 | 3.371 | 2.490–4.563 | <0.001 | NA | 1.731 | 1.083–2.767 | 0.022 |
Abbreviations: NA, not reported.
‡The values of were estimate from Kaplan‐Meier survival curves applying the method by Tierney et al. 11
The NLR cut‐off values for the OS ranged from 1.96 to 5, while for the DFS ranged from 1.96 to 4.25. Ten studies 18 , 20 , 22 , 25 , 26 , 27 , 30 , 36 , 37 , 41 reported the association between the NLR and OS, three 21 , 34 , 39 between NLR and DFS and fourteen 19 , 21 , 23 , 24 , 28 , 29 , 31 , 32 , 33 , 35 , 38 , 40 , 43 , 44 studies reported the effect of NLR on both outcomes (OS and DFS/RFS). Cut‐offs values were obtained from ROC curves, median value, X‐tile software, or with Contal and O’Quigley method or using values previously reported in the literature.
3.2. Risk of bias within studies
Seven studies 19 , 24 , 26 , 28 , 29 , 40 , 44 fully complied with REMARKS guidelines, while the remaining twenty 18 , 20 , 21 , 22 , 23 , 25 , 27 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 showed weakness in some of the parameters (Table S2).
All the studies included were adequate in at least half of the parameters analysed; hence, the ROB of these studies can be considered medium overall (Figure 2).
FIGURE 2.
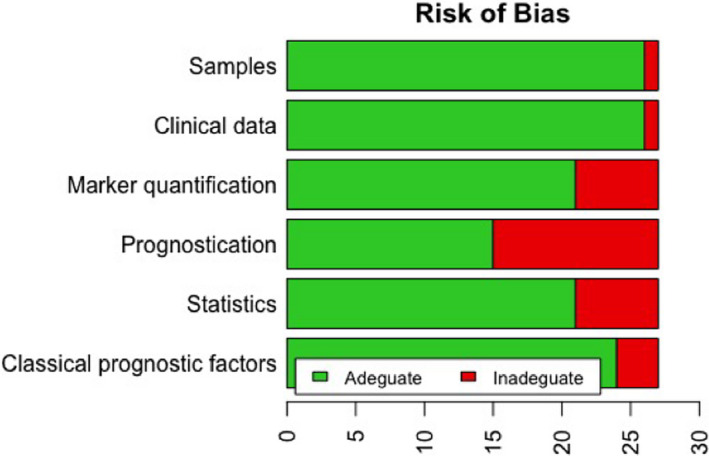
Risk of bias of studies included in the systematic review and meta‐analysis according to the REMARK guidelines
The GRADE ratings about the outcome‐centred quality of the evidence and pooled summary estimates, where applicable, have been outlined in the summary of findings table (Table S3).
The overall quality of evidence was rated as moderate for both OS and DFS. Results of the GRADE analysis indicated that there is moderate evidence to support that high pre‐treatment NLR values are associated with a worse prognosis in patients with HNSCC in terms of OS and DFS (Table S3); these results are mainly related to the design of the included studies since they are all observational studies.
3.3. NLR and prognosis in HNSCC
The meta‐analysis assessing the association between NLR and OS included 17 studies 25 , 26 , 27 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 39 , 43 , 44 , 45 , 46 , 47 with a total of 4597 patients. Meta‐analysis (Figure 3A) revealed that a higher value of pre‐treatment NLR correlates with a statistically significant decrease of OS in HNSCC patients (HR, 1.56; 95% CI: [1.35, 1.80]; p < 0.00001). A random‐effect model was used by the presence of high heterogeneity (I 2=61%). These results were also confirmed by the TSA. The graphical evaluation shows that the z‐curve crossed the monitoring boundary and APIS, revealing a significant statistical power (Figure 3b).
FIGURE 3.
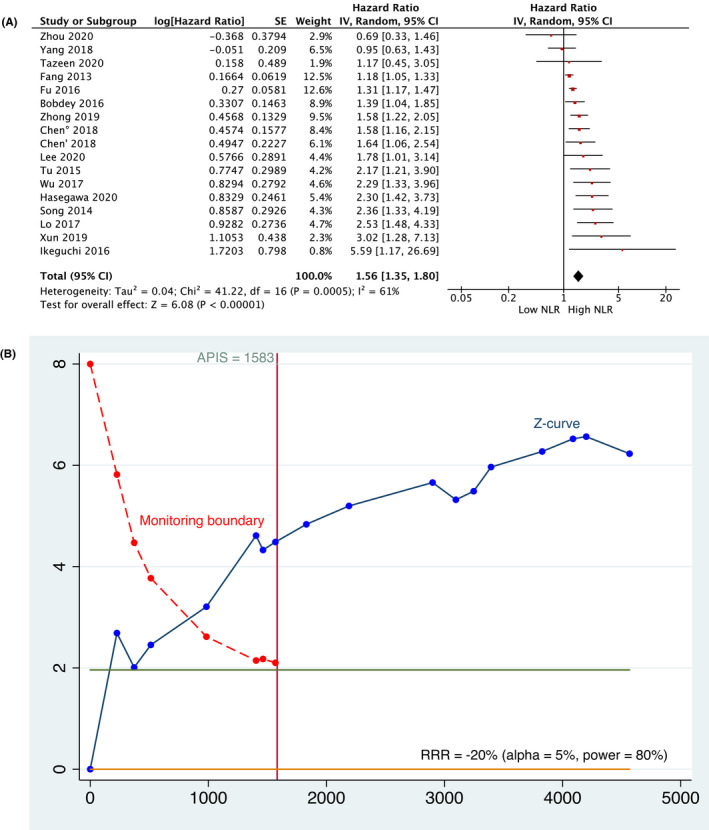
(A) Meta‐analysis and (B) TSA related to the association between NLR and Overall Survival
A total of 10 studies, 19 , 23 , 24 , 28 , 29 , 32 , 39 , 40 , 42 , 44 including 2020 patients, evaluated the prognostic role of NLR for DFS. Meta‐analysis (Figure 4A) assessed that a higher value of NLR was associated with worse survival in HNSCC patients (HR, 1.64; 95% CI: [1.30, 2.07]; p < 0.0001). The analysis was performed at random‐effect model due to the high rate of heterogeneity (I2 =69%). The graphical evaluation of TSA revealed a high statistical power as the z‐curve crossed the trial sequential monitoring boundary and the APIS (Figure 4B).
FIGURE 4.
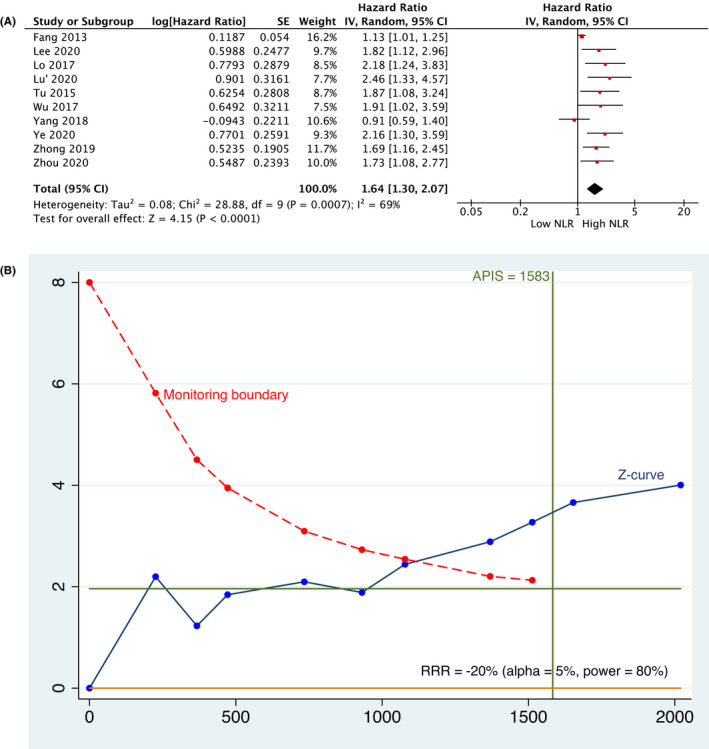
(A) Meta‐analysis and (B) TSA related to the association between NLR and Disease‐free Survival
4. DISCUSSION
Several inflammatory markers, such as NLR, PLR and LMR have been reported to be associated with clinical outcomes in patients with various types of cancer, including lung cancer, colorectal cancer, breast cancer, thyroid and so forth. 48 Furthermore, a novel systemic immune‐inflammation index (SII) based on peripheral neutrophil, platelet and lymphocyte count further enhanced the validity to predict the tumour prognosis. 40
Various potential mechanisms that may justify the prognostic role of NLR and the role of systemic inflammation in cancer biology have been hypothesized. The adaptive immune system carries out immune surveillance against cancer cells, but effective adaptive immune responses are always suppressed through several pathways. 49
Neutrophilia may inhibit the immune system by regulating the activation and the infiltration of regulatory T cells 45 and suppressing the cytolytic activity of lymphocytes and natural killer (NK) cells. 45 , 50
Neutrophils and other cells such as macrophages have been reported to secrete tumour growth‐promoting factors contributing to create a favouring microenvironment for extracellular matrix remodelling, endothelial cell migration and tumour dissociation. 7 Moreover, an elevated NLR has been associated with an increase in the peritumoural infiltration of macrophages and an increasing in IL‐17. 18 The effects of inflammatory cytokines and chemokines secreted by tumour, stromal and associated host cells in the tumour microenvironment are important in sustaining chronic inflammation (e.g. promoting and increasing the differentiation and the release of bone‐marrow neutrophils). 51
Otherwise, lymphopenia could impair the role of cell‐mediated immunity and its function of host cancer cell suppression. 46 It has been reported that increasing infiltration of lymphocytes in the tumour microenvironment is associated with a better response to cytotoxic treatment and prognosis in cancer patients. 47 Recent studies showed that the NLR may be useful in identifying patients at highest risk for neck nodal occult metastasis in tongue OSCC and orient the clinicians to the elective neck dissection, 52 as it was also demonstrated in patients with melanoma. 53
Although other systematic reviews evaluated the prognostic role of NLR in HNSCC have been performed, 54 , 55 , 56 , 57 , 58 , 59 the present meta‐analysis is more comprehensive, including the most recent findings and being the first to perform TSA for time‐to‐event outcomes, to adjust for type I and type II errors and to quantify the power of the published evidence. This is the first systematic review to include only studies with patients undergoing surgery as first treatment with or without adjuvant therapy, as differences in the treatment plan could affect both the immune system response and the prognosis, and to include only studies with primary HNSCC and, in addition we also excluded HPV +tumours to further standardize the analysed cohort. Moreover this study is the only one to assess the certainty of evidence applying the GRADE approach.
Such analysis 14 was performed to evaluate the quality of evidence and to observe the strength of recommendation obtained from the results. The quality of the evidence was determined to be moderate for the findings associated with OS and DFS. Based on our focused question (Is high NLR associated with a worse prognosis in HNSCC?) and the studies assessed, inconsistency was evaluated according to Guyatt et al. 60 Although the I2 values were high, due to different follow‐up and cut‐off values, (61% and 69% for OS and DFS respectively), only 2 studies (OS) and 1 study (DFS) have a value of HR below 1. Furthermore, only the Cis of 3 out of 17 studies for OS, and only 1 out of 10 studies for DFS cross the value of 1; additionally, there is a large overlap of CIs in both the analysis. It should be considered that the effects are small and the sample sizes are large and, therefore relatively small differences can produce a large I2 . For these reasons, it was chosen to not downgrade the inconsistency domain and it was set at no serious ROB. The imprecision domain was assessed from the sample size and its confidence intervals, which did not reveal a serious ROB. Regarding the risk of publication bias, since this study was performed without restriction regarding date of publication and the bibliography of the previous systematic reviews was hand‐revised, a low risk of publication bias was detected in the current review.
The results obtained from the meta‐analysis confirm what was previously asserted by the previous systematic reviews about the role of pre‐treatment NLR as an independent prognostic factor in terms of OS (HR, 1.56; 95% CI: [1.35, 1.80]; p < 0.00001) and DFS (HR, 1.64; 95% CI: [1.30, 2.07]; p < 0.0001). Considering the role of NLR as a heavily investigated factor by the latest meta‐ analyses performed in 2018, the authors considered appropriate to perform a TSA to control for the risk of random error in cumulative meta‐analyses and the possible presence of false positive results 61 due to the increase in the number of included studies. TSA provides the necessary sample size, monitoring and futility boundaries analogous to constructing interim monitoring boundaries for individual randomized clinical trial.
Based on TSA, the association between NLR and OS or DFS is supported by a high statistical power showing that the results are true positives crossing the threshold of statistical significance (trial sequential monitoring boundary) and reaching an adequate number of participants, or optimal information size (APIS) in both analyses; furthermore, the results supported by an adequate number of studies included in the analysis.
Although the TSA results showed high statistical power, some authors have pointed out that stating that no further studies are needed on the subject may be premature. This is because the construction of the monitoring boundaries for the meta‐analysis may be imprecise, because there is usually no control over the generation of new evidence (i.e. the adoption of discontinuation rules) unlike what happens in individual randomized clinical trial. 62 For this reason, the use of the TSA should be interpreted as a detection of false positive or false negative results in the meta‐analysis rather than as an index of no need to carry out new studies. 62
This study presents some limitations. First, all the included studies were retrospective and observational, this could lead to a potential selection bias. Furthermore, although Cho et al. 57 stated that absolute NLR cut‐off values did not seem to matter instead of that groups below and above NLR cut‐offs did show significant survival differences, the identification of a single cut‐off value could improve the prognostic performance of this biomarker and may reduce heterogeneity between studies obtaining even more precise results in the meta‐analytic statistical analysis. The eligible studies identified NLR cut‐off values using different methods, with a wide range (from 1.96 to 4.81) and it was not possible to identify the most effective cut‐off as complete data for each study are not available, but summary data for each analysis were reported. Additionally, a non‐uniform methodology was also identified in tumour staging, for this reason no subgroup analysis was performed.
5. CONCLUSION
This study reports the most recent data about the prognostic role of the NLR in HNSCC patients, confirming that a high pre‐treatment NLR is associated with a worse prognosis in terms of OS and DFS. Considering the limitations highlighted during the elaboration of this work, the high heterogeneity founded and that the results are supported by a moderate quality of scientific evidence, it is strongly recommended that future studies on this topic should be developed prospectively to make better use of human and economic resources and to confirm the true‐positive result that NLR is a prognostic factor for HNSCC.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
Pierluigi Mariani: Conceptualization; Data curation; Formal analysis; Software; Writing – original draft. Diana Russo: Formal analysis; Investigation; Software. Marco Maisto: Data curation; Investigation; Visualization. Giuseppe Troiano: Conceptualization; Data curation; Software; Writing – review & editing. Vito Carlo Alberto Caponio: Investigation; Methodology; Resources. Marco Annunziata: Formal analysis; Methodology. luigi laino: Conceptualization; Funding acquisition; Project administration; Validation; Writing – review & editing.
ETHICAL APPROVAL
Given that this is a systematic review, no ethical approval was required.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jop.13264.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
Open Access Funding provided by Universita degli Studi di Foggia within the CRUI‐CARE Agreement.
Mariani P, Russo D, Maisto M, et al. Pre‐treatment neutrophil‐to‐lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta‐analysis and trial sequential analysis. J Oral Pathol Med. 2022;51:39–51. doi: 10.1111/jop.13264
REFERENCES
- 1. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6. 10.1038/s41572-020-00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braakhuis BJM, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014;50:670‐675. 10.1016/j.oraloncology.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4. Wu P, Wu D, Zhao L, et al. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta‐analysis. Oncotarget. 2016;7:40451‐40460. 10.18632/oncotarget.9625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor‐associated neutrophils as a new prognostic factor in cancer: a systematic review and meta‐analysis. PLoS One. 2014;9:1‐10. 10.1371/journal.pone.0098259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst. 2014;106:1‐11. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review). Int J Oncol. 2016;49:857‐867. 10.3892/ijo.2016.3616 [DOI] [PubMed] [Google Scholar]
- 8. Higgins J, Green S. Cochrane collaboration. cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2008;187‐235. [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Linee guida per il reporting di revisioni sistematiche e meta‐analisi : il PRISMA Statement. Evidence. 2015;7:1‐36. [Google Scholar]
- 10. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting ‐ meta‐analysis of observational studies in epidemiology (MOOSE) group B. JAMA Neurol. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 11. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8:1‐16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216. 10.1371/journal.pmed.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG. Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst. 2018;110:803‐811. 10.1093/jnci/djy088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383‐394. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 15. Miladinovic B, Hozo I, Djulbegovic B. Trial sequential boundaries for cumulative meta‐analyses. Stata J. 2013;13:77‐91. 10.1177/1536867x1301300106 [DOI] [Google Scholar]
- 16. Chen LM, Ibrahim JG, Chu H. Flexible stopping boundaries when changing primary endpoints after unblinded interim analyses. J Biopharm Stat. 2014;24:817‐833. 10.1080/10543406.2014.901341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. J Clin Epidemiol. 2008;61:64‐75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 18. Bobdey S, Ganesh B, Mishra P, Jain A. Role of monocyte count and neutrophil‐to‐lymphocyte ratio in survival of oral cancer patients. Int Arch Otorhinolaryngol. 2017;21:21‐27. 10.1055/s-0036-1587318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee S, Kim DW, Kwon S, Kim HJ, Cha IH, Nam W. Prognostic value of systemic inflammatory markers for oral cancer patients based on the 8th edition of AJCC staging system. Sci Rep. 2020;10(1):1‐9. 10.1038/s41598-020-68991-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xun Y, Wang M, Sun H, Shi S, Guan B, Yu C. Prognostic analysis of preoperative inflammatory biomarkers in patients with laryngeal squamous cell carcinoma. Ear, Nose Throat J. 2020;99:371‐378. 10.1177/0145561319876910 [DOI] [PubMed] [Google Scholar]
- 21. Zhang B, Du W, Gan K, Fang Q, Zhang X. Significance of the neutrophil‐to‐lymphocyte ratio in young patients with oral squamous cell carcinoma. Cancer Manag Res. 2019;11:7597‐7603. 10.2147/CMAR.S211847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kao HK, Löfstrand J, Loh CYY, et al. Nomogram based on albumin and neutrophil‐to‐lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci Rep. 2018;8:1‐9. 10.1038/s41598-018-31498-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J, Hsueh CY, Cao W, Zhou L. Pretreatment lymphocyte‐to‐monocyte ratio as an independent prognostic factor for hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2018;138:734‐740. 10.1080/00016489.2018.1449965 [DOI] [PubMed] [Google Scholar]
- 24. Wu CN, Chuang HC, Lin YT, Fang FM, Li SH, Chien CY. Prognosis of neutrophil‐to‐lymphocyte ratio in clinical early‐stage tongue (cT1/T2N0) cancer. Onco Targets Ther. 2017;10:3917‐3924. 10.2147/OTT.S140800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ikeguchi M. Glasgow prognostic score and neutrophil‐lymphocyte ratio are good prognostic indicators after radical neck dissection for advanced squamous cell carcinoma in the hypopharynx. Langenbeck’s Arch Surg. 2016;401:861‐866. 10.1007/s00423-016-1453-9 [DOI] [PubMed] [Google Scholar]
- 26. Fu Y, Liu W, Ouyang D, Yang A, Zhang Q. Preoperative neutrophil‐to‐lymphocyte ratio predicts long‐term survival in patients undergoing total laryngectomy with advanced laryngeal squamous cell carcinoma. Med (United States). 2016;95:1‐6. 10.1097/MD.0000000000002689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song Y, Liu H, Gao L, et al. Preoperative neutrophil‐to‐lymphocyte ratio as prognostic predictor for hypopharyngeal squamous cell carcinoma after radical resections. J Craniofac Surg. 2015;26:e137‐e140. 10.1097/SCS.0000000000001235 [DOI] [PubMed] [Google Scholar]
- 28. Fang HY, Huang XY, Chien HT, et al. Refining the role of preoperative C‐reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma. Laryngoscope. 2013;123:2690‐2699. 10.1002/lary.24105 [DOI] [PubMed] [Google Scholar]
- 29. Tu XP, Qiu QH, Chen LS, et al. Preoperative neutrophil‐to‐lymphocyte ratio is an independent prognostic marker in patients with laryngeal squamous cell carcinoma. BMC Cancer. 2015;15:1‐7. 10.1186/s12885-015-1727-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasegawa T, Iga T, Takeda D, et al. Neutrophil‐lymphocyte ratio associated with poor prognosis in oral cancer: a retrospective study. BMC Cancer. 2020;20:1‐9. 10.1186/s12885-020-07063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Wang S, Song X, et al. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. Onco Targets Ther. 2016;9:7177‐7185. 10.2147/OTT.S113307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lo WC, Wu CT, Wang CP, et al. The pretreatment neutrophil‐to‐lymphocyte ratio is a prognostic determinant of T3–4 hypopharyngeal squamous cell carcinoma. Ann Surg Oncol. 2017;24:1980‐1988. 10.1245/s10434-017-5865-8 [DOI] [PubMed] [Google Scholar]
- 33. de Almeida JR, Yao CMKL, Ziai H, et al. Postoperative wound infections, neutrophil‐to‐lymphocyte ratio, and cancer recurrence in patients with oral cavity cancer undergoing surgical resection. Oral Oncol. 2019;97:23‐30. 10.1016/j.oraloncology.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 34. Chen H, Song S, Zhang L, Dong W, Chen X, Zhou H. Preoperative platelet‐lymphocyte ratio predicts recurrence of laryngeal squamous cell carcinoma. Futur Oncol. 2020;16:209‐217. 10.2217/fon-2019-0527 [DOI] [PubMed] [Google Scholar]
- 35. Chen S, Guo J, Feng C, Ke Z, Chen L, Pan Y. The preoperative platelet‐lymphocyte ratio versus neutrophil‐lymphocyte ratio: which is better as a prognostic factor in oral squamous cell carcinoma? Ther Adv Med Oncol. 2016;8:160‐167. 10.1177/1758834016638019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer. 2018;18:1‐9. 10.1186/s12885-018-4730-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen F, Lin L, Liu F, et al. Three prognostic indexes as predictors of response to adjuvant chemoradiotherapy in patients with oral squamous cell carcinoma after radical surgery: a large‐scale prospective study. Head Neck. 2018:41(2):301‐308. 10.1002/hed.25495 [DOI] [PubMed] [Google Scholar]
- 38. Lu Z, Yan W, Liang J, et al. Nomogram based on systemic immune‐inflammation index to predict survival of tongue cancer patients who underwent cervical dissection. Front Oncol. 2020;10:1‐11. 10.3389/fonc.2020.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu HJ, Tseng SW, Peng CY, et al. Predictors of early progression after curative resection followed by platinum‐based adjuvant chemoradiotherapy in oral cavity squamous cell carcinoma. Postgrad Med. 2021;133:377‐384. 10.1080/00325481.2020.1809869 [DOI] [PubMed] [Google Scholar]
- 40. Zhou S, Yuan H, Wang J, et al. Prognostic value of systemic inflammatory marker in patients with head and neck squamous cell carcinoma undergoing surgical resection. Futur Oncol. 2020;16:559‐571. 10.2217/fon-2020-0010 [DOI] [PubMed] [Google Scholar]
- 41. Szilasi Z, Jósa V, Zrubka Z, et al. Neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios as prognostic markers of survival in patients with head and neck tumours—results of a retrospective multicentric study. Int J Environ Res Public Health. 2020;17:1742. 10.3390/ijerph17051742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ye J, Liao B, Jiang X, et al. Prognosis value of platelet counts, albumin and neutrophil‐lymphocyte ratio of locoregional recurrence in patients with operable head and neck squamous cell carcinoma. Cancer Manag Res. 2020;12:731‐741. 10.2147/CMAR.S234618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tazeen S, Prasad K, Harish K, Sagar P, Kapali AS, Chandramouli S. Assessment of pretreatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in prognosis of oral squamous cell carcinoma. J Oral Maxillofac Surg. 2020;78:949‐960. 10.1016/j.joms.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 44. Zhong B, Deng D, Du JT, Chen F, Liu YF, Liu SX. Prognostic value of the preoperative neutrophil to lymphocyte ratio in patients with sinonasal squamous cell carcinoma. Cancer Manag Res. 2019;11:9733‐9741. 10.2147/CMAR.S231085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mandó P, Rizzo M, Roberti MP, et al. High neutrophil to lymphocyte ratio and decreased CD69+NK cells represent a phenotype of high risk in early‐stage breast cancer patients. Onco Targets Ther. 2018;11:2901‐2910. 10.2147/OTT.S160911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22‐28. 10.1097/01.mpa.0000188305.90290.50 [DOI] [PubMed] [Google Scholar]
- 47. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor‐infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node‐positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin‐based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860‐867. 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 48. Lang BHH, Ng CPC, Au KB, Wong KP, Wong KKC, Wan KY. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg. 2014;38:2605‐2612. 10.1007/s00268-014-2630-z [DOI] [PubMed] [Google Scholar]
- 49. Islam MM, Faruque MRI, Islam MT. A compact disc‐shaped printed antenna using parasitic element on ground plane for super wideband applications. Appl Comput Electromagn Soc J. 2016;31:960‐969. 10.1038/ni.2703.Innate [DOI] [Google Scholar]
- 50. Chen Y, Yan H, Wang Y, Shi Y, Dai G. Significance of baseline and change in neutrophil‐to‐lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep. 2017;7:1‐9. 10.1038/s41598-017-00859-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coffelt SB, Wellenstein MD, De Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431‐446. 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 52. Abbate V, Dell’Aversana Orabona G, Salzano G, et al. Pre‐treatment Neutrophil‐to‐Lymphocyte Ratio as a predictor for occult cervical metastasis in early stage (T1–T2 cN0) squamous cell carcinoma of the oral tongue. Surg Oncol. 2018;27:503‐507. 10.1016/j.suronc.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 53. Robinson AV, Keeble C, Lo MCI, et al. The neutrophil–lymphocyte ratio and locoregional melanoma: a multicentre cohort study. Cancer Immunol Immunother. 2020;69:559‐568. 10.1007/s00262-019-02478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang L, Huang Y, Zhou L, Dai Y, Hu G. High pretreatment neutrophil‐to‐lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma : systematic review and meta‐analysis. Head Neck. 2018;2019:1525‐1535. 10.1002/hed.25583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu Y, Wang H, Yan A, et al. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: a meta‐analysis. BMC Cancer. 2018;18:1‐9. 10.1186/s12885-018-4230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mascarella MA, Mannard E, Silva SD, Zeitouni A. Neutrophil‐to‐lymphocyte ratio in head and neck cancer prognosis: a systematic review and meta‐analysis. Head Neck. 2018;40:1091‐1100. 10.1002/hed.25075 [DOI] [PubMed] [Google Scholar]
- 57. Cho JK, Kim MW, Choi IS, et al. Optimal cutoff of pretreatment neutrophil‐to‐lymphocyte ratio in head and neck cancer patients: a meta‐analysis and validation study. BMC Cancer. 2018;18:1‐9. 10.1186/s12885-018-4876-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takenaka Y, Oya R, Kitamiura T, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in head and neck cancer: a meta‐analysis. Head Neck. 2018;40:647‐655. 10.1002/hed.24986 [DOI] [PubMed] [Google Scholar]
- 59. Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil‐to‐lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta‐analysis. Head Neck. 2018;40:2546‐2557. 10.1002/hed.25324 [DOI] [PubMed] [Google Scholar]
- 60. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. rating the quality of evidence ‐ Inconsistency. J Clin Epidemiol. 2011;64:1294‐1302. 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 61. Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Control Clin Trials. 1997;18:580‐593. 10.1016/S0197-2456(97)00051-2 [DOI] [PubMed] [Google Scholar]
- 62. Miladinovic B, Kumar A, Hozo I, Mahony H, Djulbegovic B. Trial sequential analysis may be insufficient to draw firm conclusions regarding statistically significant treatment differences using observed intervention effects: a case study of meta‐analyses of multiple myeloma trials. Contemp Clin Trials. 2013;34:257‐261. 10.1016/j.cct.2012.12.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


