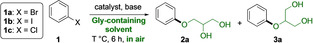
Table 1.
Optimization of Ullmann‐type C−O bond formation between halobenzene 1 and Gly of the corresponding eutectic mixture to give adducts 2 a and 3 a.[a]
|
| ||||||
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
Entry |
Solvent |
Catalyst (mol %) |
Base (equiv.) |
T [°C] |
2a+3a yield[b] [%] |
2a/3a [c] |
|
1 |
ChCl/Gly[d] |
CuI (10) |
K2CO3 (2) |
60 |
–[e] |
– |
|
2 |
ChCl/Gly[d] |
CuI (10) |
K2CO3 (2) |
80 |
98 |
77 : 23 |
|
3 |
ChCl/Gly[d] |
CuI (5) |
K2CO3 (1) |
80 |
98 |
80 : 20 |
|
4 |
ChCl/Gly[f] |
CuI (5) |
K2CO3 (1) |
80 |
98 |
80 : 20 |
|
5 |
ChCl/Gly[g] |
CuI (5) |
K2CO3 (1) |
100 |
30[e] |
80 : 20 |
|
6 |
ChCl/Gly[d] |
CuI (5) |
– |
80 |
NR[h] |
– |
|
7 |
ChCl/Gly[d] |
– |
K2CO3 (1) |
100 |
NR[h] |
– |
|
8 |
ChCl/Gly[d] |
CuI (5) |
Cs2CO3 (1) |
80 |
98 |
78 : 22 |
|
9 |
ChCl/Gly[d] |
CuI (5) |
tBuOK (1) |
80 |
98 |
77 : 23 |
|
10 |
ChCl/Gly[d] |
CuO (5) |
K2CO3 (1) |
80 |
90 |
78 : 22 |
|
11 |
ChCl/Gly[d] |
CuCl2 (5) |
K2CO3 (1) |
80 |
98 |
80 : 20 |
|
12 |
ChCl/Gly[d] |
Pd(OAc)2 (5) |
K2CO3 (1) |
100 |
–[i] |
– |
|
13 |
Gly |
CuI (5) |
K2CO3 (1) |
100 |
75 |
76 : 24 |
|
14 |
ChCl/Gly[j] |
CuI (5) |
K2CO3 (1) |
80 |
96 |
80 : 20 |
|
15 |
ChCl/Gly[k] |
CuI (5) |
K2CO3 (1) |
80 |
98 |
80 : 20 |
|
16 |
Pro/Gly |
CuI (5) |
K2CO3 (1) |
100 |
– |
– |
|
17 |
betaine/Gly |
CuI (5) |
K2CO3 (1) |
100 |
20[l] |
78 : 22 |
[a] Reaction conditions: 1.0 g DES or 1 mL Gly per 1.0 mmol of 1 a–c; DES: ChCl/Gly (1 : 2, 1 : 1, or 1 : 3 mol mol−1); l‐proline (Pro)/Gly (2 : 5 mol mol−1); betaine/Gly (1 : 2 mol mol−1). [b] The yields reported are for products isolated and purified by column chromatography. [c] Calculated by 1H NMR spectroscopy of the crude reaction mixture using an internal standard technique (NMR internal standard: CH2Br2). [d] X=Br. [e] Reaction time: 24 h. [f] X=I. [g] X=Cl. [h] NR=no reaction. [i] Biphenyl was the only adduct isolated (98 % yield). [j] 1 : 1 mol mol−1. [k] 1 : 3 mol mol−1. [l] After 24 h: 55 % yield.