FIGURE 4.
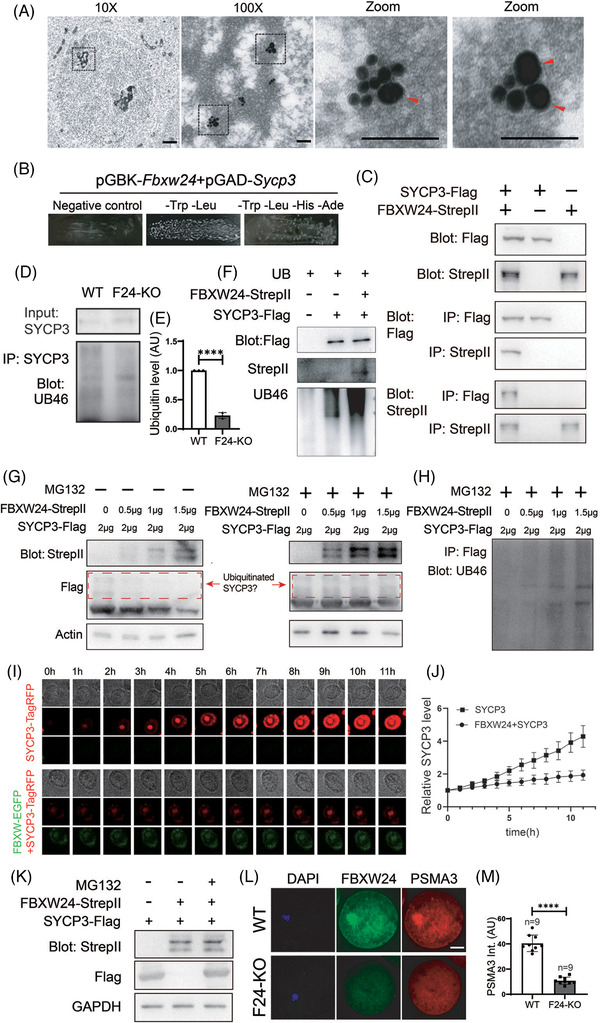
FBXW24 directly binds and ubiquitinates SYCP3. (A) Immuno‐EM shows that in the chromatin regions of meiotic cells in the 16.5 DPC genital ridge, some SYCP3 signals localized closely to FBXW24 (<20 nm). One region (dot‐line square) from a 10x image was photographed at 100x to show the overall signal within the chromatin region; two regions (dot‐line square) from the 100x image were further magnified to show the close adjacency between SYCP3 and FBXW24. SYCP3 and FBXW24 were detected by secondary antibodies conjugated with 15 nm gold (small dots) and 35 nm gold (big dots, arrow‐pointed), respectively. (B) Yeast‐two‐hybridization (Y2H) assay shows that FBXW24 can directly bind SYCP3. (C) Co‐IP between in‐vitro purified FBXW24 and SYCP3 shows that FBXW24 directly binds SYCP3. 1 μg SYCP3‐Flag protein and/or FBXW24‐StrepII protein were/was used in each reaction. (D and E) SYCP3 IP, western blot and quantification demonstrate that Fbxw24 knockout significantly decreased SYCP3 ubiquitination level. (F) In‐vitro ubiquitination assay showed that in the presence of ubiquitin and other components (Table S5), FBXW24 can significantly increase the SYCP3 ubiquitination level. 1 μg SYCP3‐Flag protein and/or FBXW24‐StrepII protein were/was in each reaction. (G and H) 293T cells were transfected with Fbxw24‐StrepII and Sycp3‐Flag plasmid (Amounts of plasmid used were in the image), and western blot was done under two conditions. Left of G, in the absence of MG132, as the FBXW24 level increased, the SYCP3 level gradually decreased. Right of G and H, in the presence of MG132, as the FBXW24 level increased, the SYCP3 level remained unchanged, while ubiquitinated SYCP3 gradually increased. Red dot‐line square labelled probable ubiquitinated SYCP3. (I and J) SYCP3 titer were first added into the Sf9 medium to be expressed to a medium level (at about Day 1.5), then FBXW24 titer was added, and SYCP3 intensity almost remained unchanged; In contrast, in the sf9 medium without FBXW24 supplement, SYCP3 intensity keep raising, and big SYCP3 aggregates kept increasing. (K) Western blot shows that FBXW24 expression substantially reduced SYCP3 level; once proteasome activity was inhibited with MG132, SYCP3 level is recovered. (L and M) In WT GV oocytes, PSMA3, a sub‐unit of the proteasome, was enriched within nuclear chromatin and highly overlapped with FBXW24; in Fbxw24‐KO GV oocytes, PSMA3 did not show any abundance within chromatin. For all plasmids except B, Sycp3 is fused to TagRFP and Flag, and Fbxw24 is fused to EGFP and Strep II. To save space, SYCP3‐TagRFP‐Flag was shortened as "SYCP3‐TagRFP" or "SYCP3‐Flag", and FBXW24‐EGFP‐stepII was shortened as "FBXW24‐EGFP" or "FBXW24‐Strep II" as needed. Scale bar for 10X image in A, 1 μm; for 100X and zoom images in A, 100 nm. Scale bar for other panels, 20 μm. Actin or GAPDH was used as a loading control. ****, p < 0.0001. AU, arbitrary unit
