FIGURE 5.
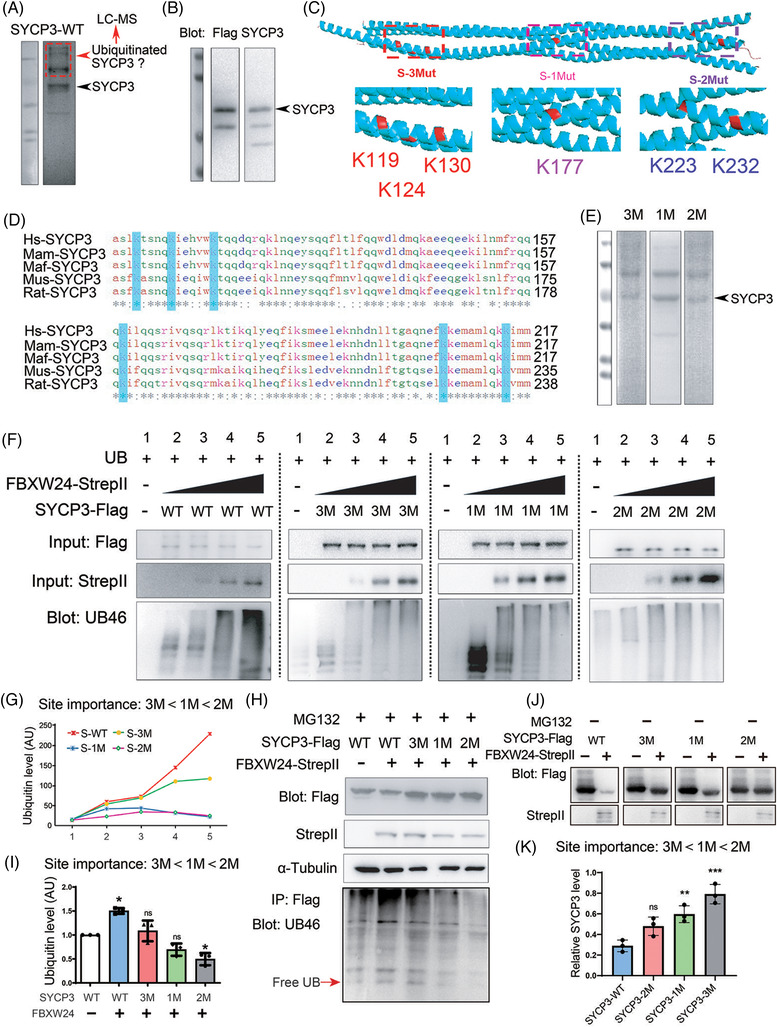
Characterization of key sites in SYCP3 for ubiquitination. (A) Purified WT SYCP3‐TagRFP‐Flag protein was subjected to SDS‐PAGE and Coomassie staining. The stained gel shows that the purified WT SYCP3 demonstrated multiple bands; the lower band (arrow‐pointed) corresponded to the expected size band, and the other upper bands were cut altogether for the characterization of ubiquitination sites within SYCP3 through mass spectrometry (Figure S11A–I and Table S4). (B) The expected right‐size band in A was verified through Flag and SYCP3 western blots (arrow‐pointed). (C) Based on the known SYCP3 structure model, K119, K124, K130, K177, K223, and K232, which are on the surface of the SYCP3 tetramer, were subdivided into three groups according to their positional proximity, and three non‐ubiquitinated mutants were made: K119A, K124A, and K130A – simplified as S‐3 M, K177A – simplified as S‐1 M, and K223A and K232A – simplified as S‐2 M. (D) Protein sequence alignment among SYCP3 from Homo sapiens (Hs), Macaca mulatta (Mam), Macaca fascicularis (Maf), Mus musculus (Mus), and Rattus norvegicus (Rat) shows that all six selected ubiquitination sites are conserved. (E) SDS‐PAGE and Coomassie staining demonstrate that the purified SYCP3 mutant proteins also showed multiple bands; the right‐size band (arrow‐pointed) is at similar positions to SYCP3 WT protein. (F) In vitro dose‐dependent ubiquitination assay shows that each mutant showed decreased ubiquitination, but the extent of this reduction differed for these three mutants. SYCP3 WT and each mutant reaction were separated by a vertical dot‐line. 2 μg SYCP3 WT or each mutant protein was used in each reaction, 0 μg, 0.5 μg, 1 μg, 1.5 μg, or 2 μg FBXW24 protein was used in each transfection from left to right. (G) Quantification of F shows that the site importance ranking for ubiquitination appeared to be 3 M < 1 M < 2 M. (H and I) In vivo side‐by‐side comparative ubiquitination assay in the presence of MG132 also illustrates that the site importance ranking for ubiquitination appeared to be 3 M < 1 M < 2 M. 2 μg Sycp3 and/or Fbxw24 plasmid were/was used in each transfection. (J) In vivo side‐by‐side comparison of FBXW24‐dependent SYCP3 degradation among WT, 3 M, 1 M and 2 M. The degradation extent is also 3 M < 1 M < 2 M. 2 μg Sycp3 and/or Fbxw24 plasmid were/was used in each transfection. α‐tubulin was used as a loading control. *, p < 0.05; **, p < 0.01; ***, p < 0.001. AU, arbitrary unit
