FIGURE 6.
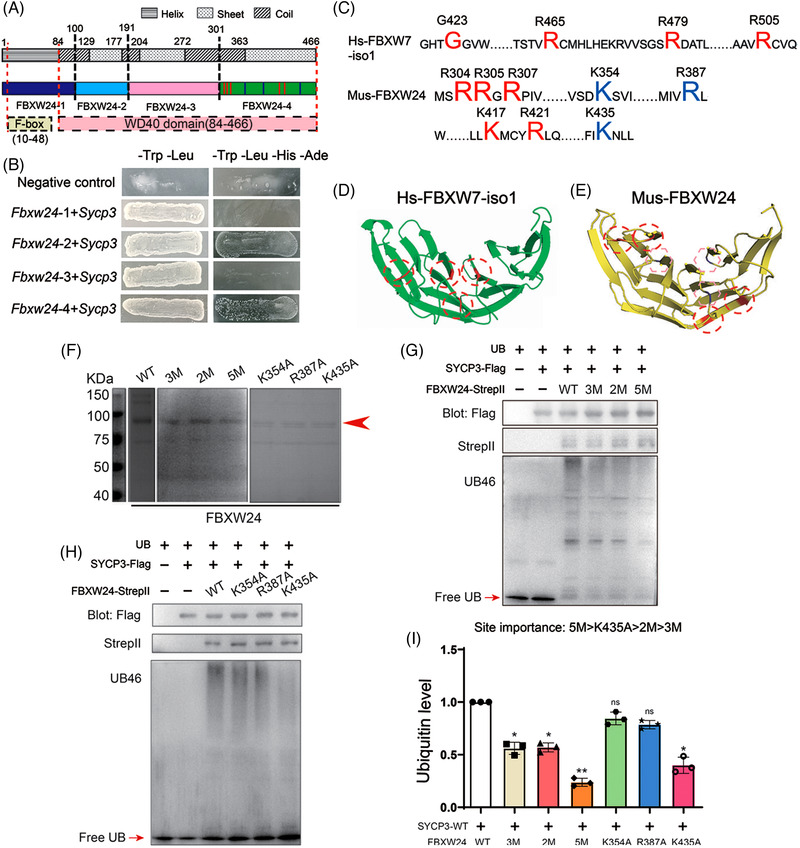
Characterization of key sites in FBXW24 required for SYCP3 ubiquitination. (A) I‐Tasser was used to predict the FBXW24 structure and FBXW24 was sub‐divided into four parts based on its secondary structure. FBXW24‐1 (1–99 AA, dark blue) is primarily α‐helix; FBXW24‐2 (100–191 AA, light blue), FBXW24‐3 (192–300 AA, pink), and FBXW24‐4 (301–466 AA, green) are mostly β‐sheet; and residues between them are primarily coils. Predicted F‐box‐like, from 10–48 AA, and WD40 domain, from 84–486 AA, was also mapped. (B) Y2H showed that FBXW24‐2 or FBXW24‐4 could directly bind SYCP3 through Y2H. (C–E) I‐Tasser showed that the R‐rich C‐terminal region of FBXW24 is structurally similar to the R & K‐rich C‐terminal region of FBXW7. We focused on two groups of R & K‐rich sites (red type in C) within FBXW24‐4 (red dot‐line circles in E) and made three FBXW24 mutants: R304A, R305A, and R307A – simplified as 3 M; K417A and R421A – simplified as 2 M; and R304A, R305A, R307A, K417A, and R421A – simplified as 5 M. We also selected three R & K sites (violet type in C) within FBXW24‐4 (pink dot‐line circles in E) that are spatially similar to the enzymatic sites (red type in C) in the C‐terminal WD domain of human FBXW7‐iso1 (isoform 1, red dot‐line circles in D), and made another three mutants: K354A, R387A, and K435A. (F) SDS‐PAGE and coomassie staining demonstrate the purity of WT, 3 M, 2 M, 5 M, K354A, R387A, and K435A (arrow‐pointed). (G) In vitro side‐by‐side comparative ubiquitination assay shows that all three mutants (3 M, 2 M, and 5 M) have decreased ubiquitinating capacity. 1 μg SYCP3‐Flag protein and/or FBXW24‐StrepII protein were/was used in each reaction. (H) In vitro side‐by‐side comparative ubiquitination assay shows that among K354A, R387A, and K435A, only K435A has significantly decreased ubiquitinating capacity. 1 μg SYCP3‐Flag protein and/or FBXW24‐StrepII protein were/was used in each reaction. (I) Quantification of G and H showed that the site importance is 5 M > K435A > 2 M > 3 M. Different lower‐case letters above the graph column indicate significant differences. Free ubiquitin was arrow‐pointed. AU, arbitrary unit
