Figure 1.
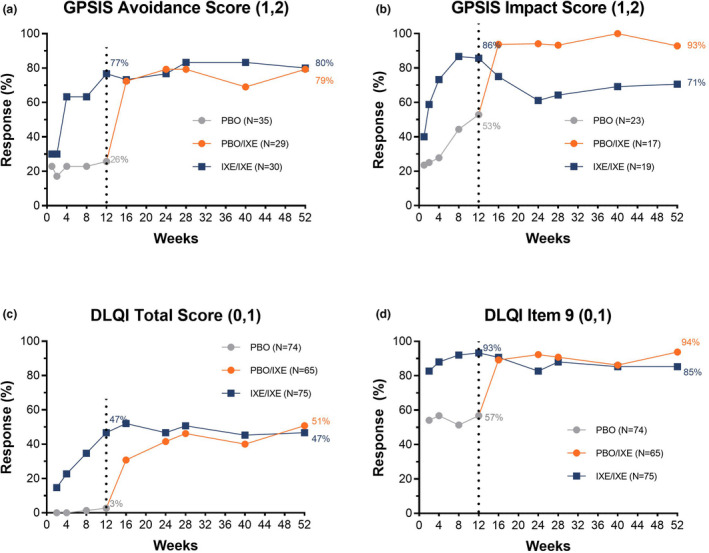
GPSIS‐Avoidance (1,2), and GPSIS‐Impact (1,2) outcomes, along with DLQI (0,1) and DLQI item 9 (0,1) responses rates, through Week 52 of IXORA‐Q (non‐responder imputation [based on patients with Mx + Px1 for GPSIS‐impact]) including blinded T2T and open‐label T2T periods. (a) Proportion of patients who reported that they never (1) or rarely (2) avoided sexual activity due to GenPs (GPSIS‐Avoidance [1,2]) [based on patients with GPSIS‐avoidance score ≥3 at baseline]; (b) Proportion of patients who reported a very low or not at all (1) or low (2) level of worsening of GenPs symptoms following sexual activity (GPSIS‐Impact [1,2]) [based on patients with GPSIS‐avoidance score ≥3 at baseline]; (c) Proportion of patients achieving a response of (0,1) in DLQI total score indicating no effect on HRQoL; (d) Proportion of patients achieving a response of (0,1) on DLQI item 9, indicating ‘no’ (0) or ‘a little’ (1) sexual difficulties due to skin. The dotted line separates the double‐blind treatment period (Weeks 0–12) and the open‐label treatment period (Weeks 12–52).
