Table 1.
Optimization of the Friedel–Crafts spirocyclization reaction.[a]
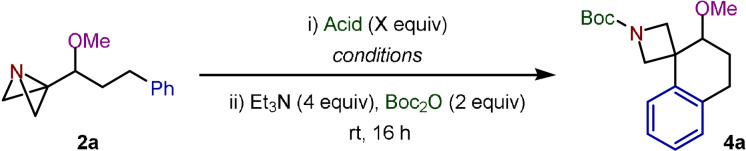
|
Entry |
Acid |
X equiv |
Conditions |
4 a: % Yield[a] |
|---|---|---|---|---|
|
1 |
In(OTf)3 |
2.00 |
CH2Cl2, 0 °C–rt, 4 h |
0 |
|
2 |
BF3⋅OEt2 |
1.10 |
CH2Cl2, 0 °C, 1.5 h |
16 |
|
3 |
TFA |
2.00 |
CH2Cl2, 0 °C, 1.5 h |
7 (52)[b] |
|
4 |
TfOH |
2.00 |
CH2Cl2, 0 °C, 1.5 h |
24 |
|
5 |
HPF6 |
1.05 |
CH2Cl2, 0 °C, 2 h |
37 |
|
6 |
HBF4 |
1.05 |
CH2Cl2, 0 °C, 1 h |
43 |
|
7 |
HBF4 |
0.50 |
CH2Cl2, 0 °C–rt, 6 h |
20 |
|
8 |
HBF4 |
1.50 |
CH2Cl2, 0 °C, 1 h |
41 |
|
9 |
HBF4 |
1.05 |
CHCl3, 0 °C, 1 h |
64 (63) [c] |
|
10 |
HBF4 |
0 |
CHCl3, 0 °C, 1 h |
0 (85)[d] |
All reactions were carried out using 2 a (0.10 mmol). [a] Yields were determined by 1H NMR analysis using dibromomethane as an internal standard after protection of the amine intermediate with Boc2O. [b] Yield of product from the intermolecular addition of trifluoroacetate to ABB. [c] 0.2 mmol scale. Isolated yield. [d] Returned 2 a.
