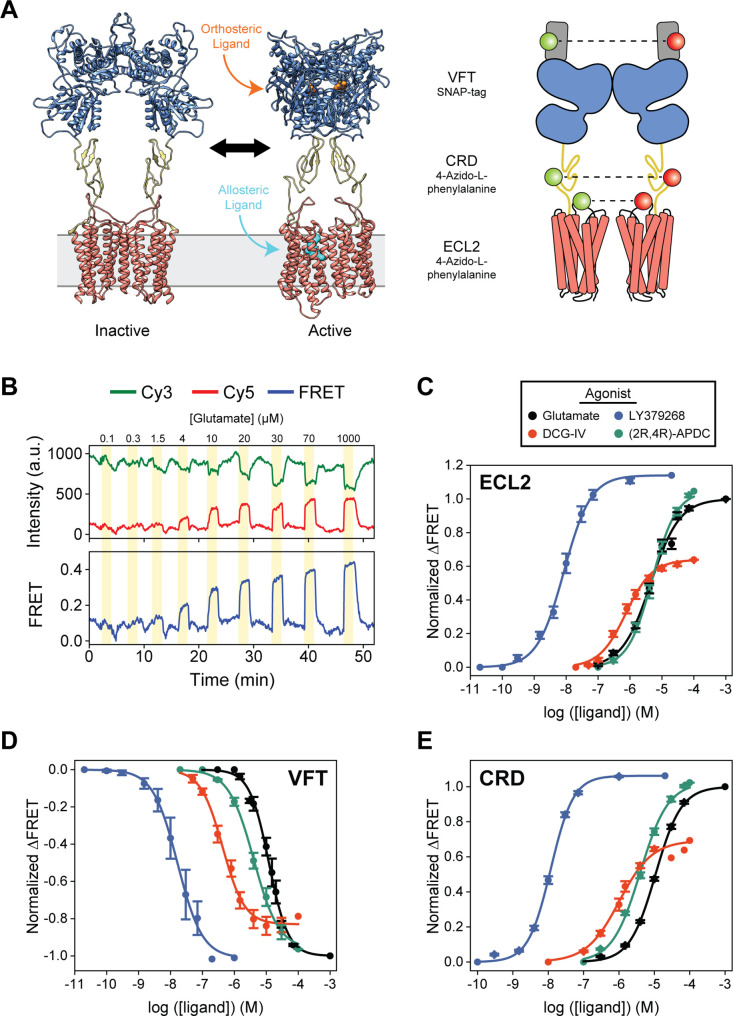
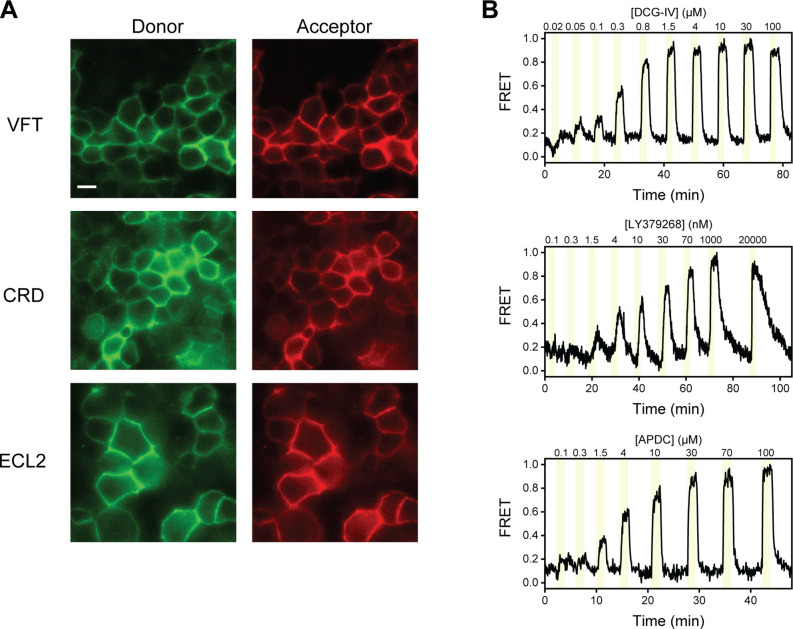
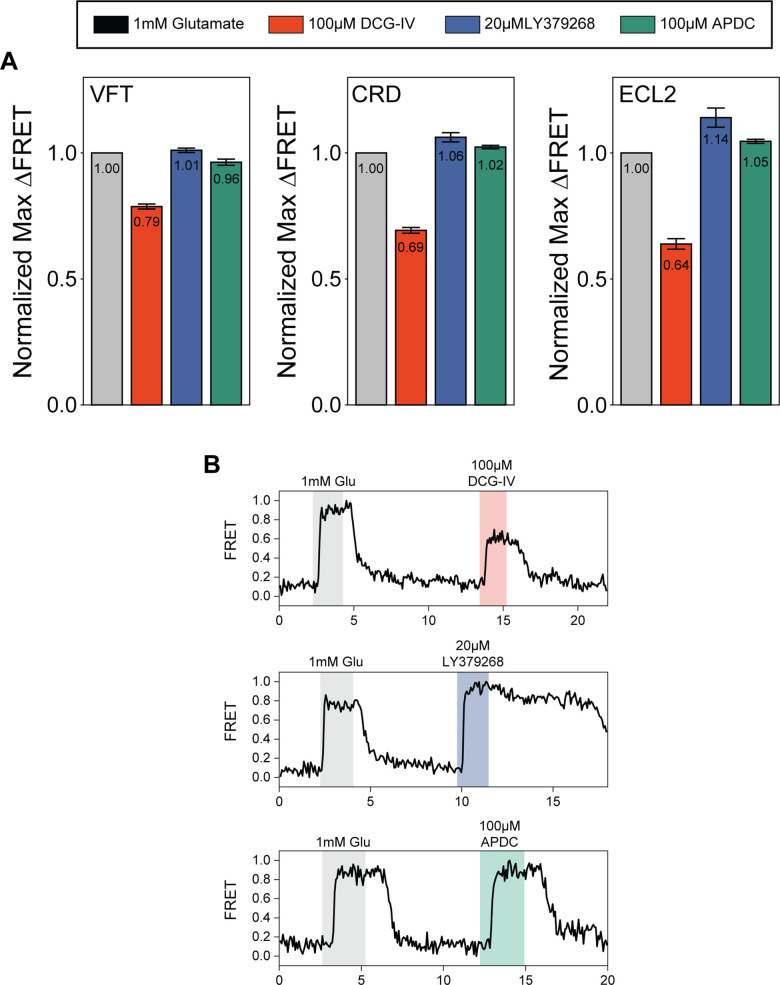
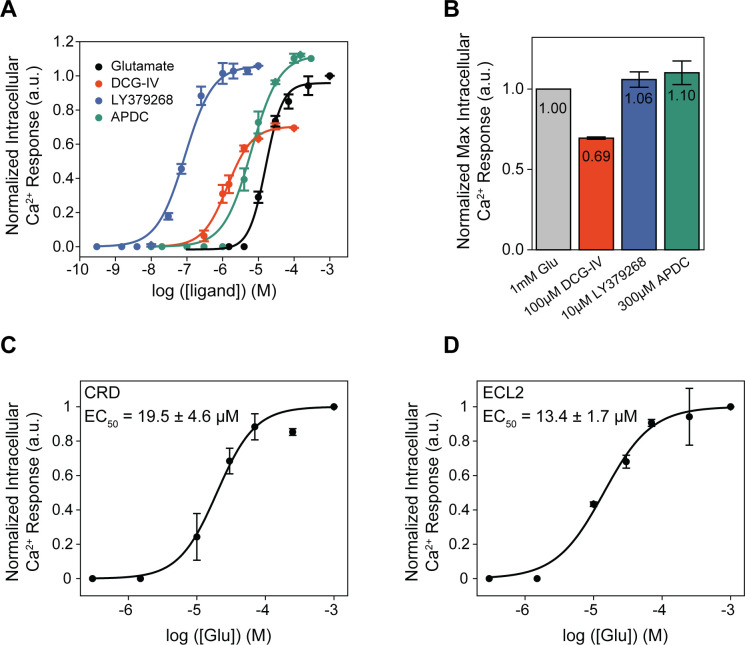
Figure 1. Agonist-induced structural change measured at each domain using conformational fluorescence resonance energy transfer (FRET) sensors.
(A) Full-length cryo-EM structures of inactive (7EPA) and fully active (7E9G) metabotropic glutamate receptor 2 (mGluR2; human) and schematic illustrating fluorophore placement for each inter-domain sensor. (B) Representative normalized live-cell FRET trace from glutamate titration experiment on HEK293T cells expressing azi-extracellular loop 2 (azi-ECL2). Data was acquired at 4.5 s time resolution. Dose-response curves from live-cell FRET orthosteric agonist titration experiments using (C) azi-ECL2, (D) N-terminal SNAP-tag labeled mGluR2 (SNAP-m2), and (E) azi-cysteine-rich domain (azi-CRD). Data is acquired from individual cells and normalized to 1 mM glutamate response. Data represents mean ± SEM of responses from individual cells from at least three independent experiments. Total number of cells examined, mean half-maximum effective concentration (EC50), mean max response, and errors are listed in Tables 1–2.