FIGURE 1.
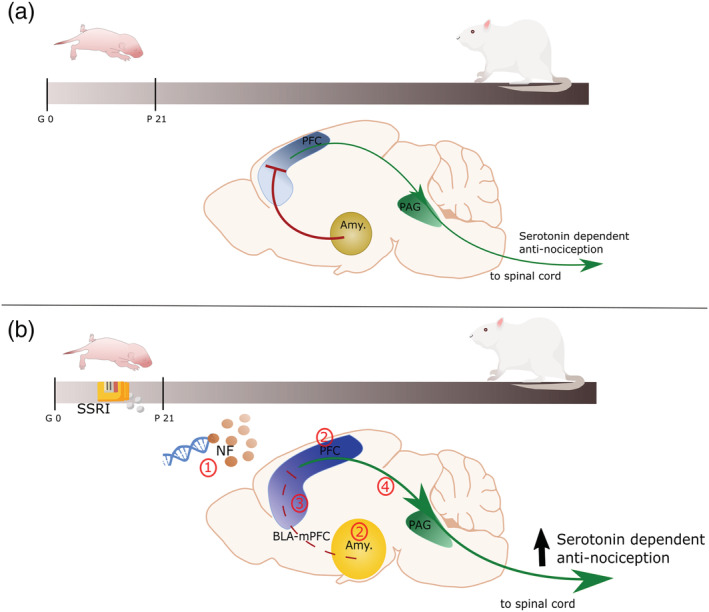
Schematic representation of the role of the PFC and amygdala roles on nociceptive processing in normal animals (a) and after early‐life SSRI exposure (b). (a) In normal condition, PVA interneurons connect the amygdala to the PFC, and with reciprocal connection form an important part of the nociceptive network in the adult rat. The PVA interneurons inhibit the pyramidal neurons of the PFC, which in turn, via the periaqueductal grey, results in a reduced serotonin‐dependent antinociceptive modulation of the spinal cord pain gate. (b) Upon early life SSRI exposure, adult animals present (1) epigenetic alteration of genes involved in cytoskeleton maturation and synaptic development, affected neurotrophic factors and serotonin levels in the PFC, (2) intra‐hyperconnectivity in the amygdala and the PFC, and (3) a diminished PVA interneurons reactivity leading to disinhibition of the PFC by the amygdala. Reduced PFC feedforward inhibition is the results of disrupted inhibitory and excitatory balance in the PVA interneurons, the monosynaptic connection of the BLA with the pyramidal neurons, and other inputs into the pyramidal neurons. (4) Consequently, PFC outputs to the spinal cord via PAG are strengthened, resulting in enhanced serotonin‐dependent antinociceptive input. Amy.: amygdala; BLA: basolateral amygdala; BLA‐PFC: network of neurons including (1) output of the BLA into PVA interneurons and pyramidal neurons, (2) input into pyramidal neurons from other cortical areas and (3) pyramidal neurons; NF: neurotrophic factors PAG: periaqueductal grey; PFC: prefrontal cortex; PVA: parvalbumin GABAergic; SSRI: selective serotonin reuptake inhibitors
