FIGURE 2.
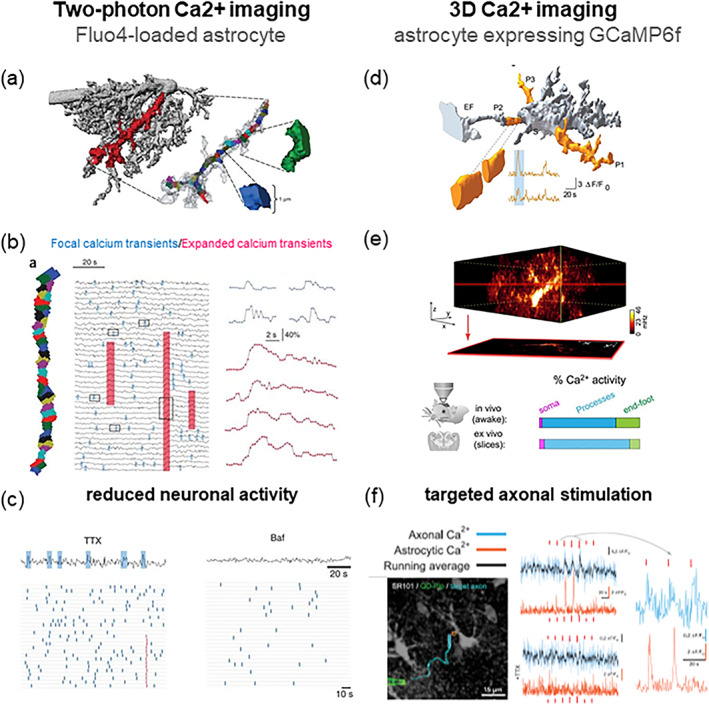
Innovative methods developed by our group to study Ca2+ dynamics in astrocytes and their dependency on neuronal activity. We monitored Ca2+ elevations in astrocytic processes by high‐resolution two‐photon Ca2+ imaging in the hippocampal dentate molecular layer (HDML) of the adult mouse. In the first study (Di Castro et al., 2011; left panels) we used the green Ca2+ indicator Fluo4 loaded into single astrocytes by the whole‐cell patch‐clamp technique. (a) Cell‐impermeant Texas red‐dextran was used to assess astrocyte morphology. An astrocytic process fully lying in the focal plane was selected for Ca2+ imaging and segmented in 1μm2 sub‐regions for Ca2+ analysis. (b) The calcium events observed in a process, were subdivided in two distinct groups: Focal events (small transients, limited to 1‐few sub‐regions, blue) and expanded events (high amplitude events, spanning several sub‐regions, red). (c) The block of action potential firing by TTX application reduced Ca2+ activity in astrocyte processes by affecting primarily expanded events. Bafilomycin A1(Baf), which depresses transmitter release by progressively depleting recycling vesicles of their transmitter content, abolished both focal and expanded Ca2+ transients in the astrocyte. In a second study (Bindocci et al., 2017; right panels) we developed a new method for 3D two‐photon Ca2+ imaging in astrocytes that selectively expressed the genetically encoded Ca2+ indicator (GECI) GCaMP6f. (d) Analysis of 3D Ca2+ dynamics was performed on segments (about 3 μm in diameter) of the reconstructed 3D core morphology of the astrocyte. (e) by rapidly scanning at least 30 individual focal planes, we could reconstruct endogenous Ca2+ dynamics in the entire volume of individual astrocytes, both in situ and in vivo in the awake, head‐fixed mouse. We found that most of the Ca2+ activity (in %) occurs in processes, is less frequent in end‐feet, and is very infrequent in the cell soma. (f) in dual color Ca2+ imaging experiments, we could show that HDML astrocytes respond to minimal stimulation of contiguous PP axons with time‐locked focal Ca2+ elevations (azur: Axonal Ca2+; orange: Astrocyte Ca2+) in a very small portion of their volume, and that TTX abolishes both axonal and astrocytic Ca2+ responses. The authors hold the copyright to reproduce panels (a), (b) and (c) Di Castro et al, Nature Neuroscience 2011 and Habbas et al., Cell 2015. Panels (d), (e) and (f) in Figure 2 are “Reprinted/adapted from Bindocci E, Savtchouk I, Liaudet N, Becker D, Carriero G, Volterra A. Three‐dimensional Ca2+ imaging advances understanding of astrocyte biology. Science. 2017 May 19; 356 (6339): eaai8185. doi: 10.1126/science.aai8185. © The authors, some rights reserved; exclusive licensee AAAS. Distributed under a CC BY‐NC 4.0 license http://creativecommons.org/licenses/by‐nc/4.0/
