FIGURE 1.
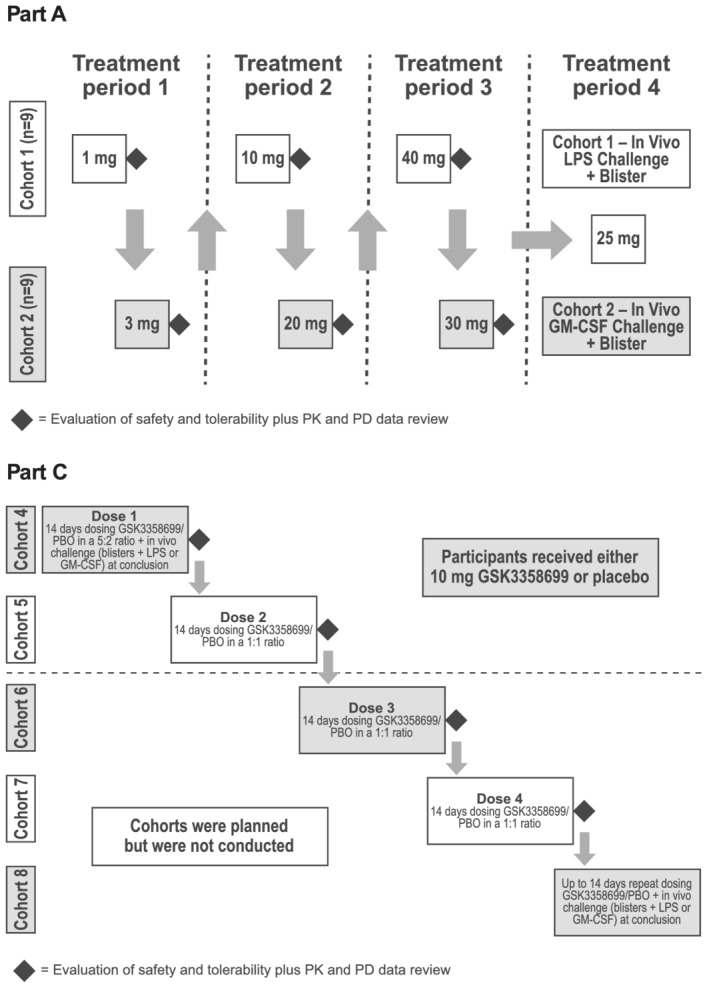
Dosing schematic. In part A of the study, two interlocking cohorts of participants (cohorts 1 and 2) received GSK3358699 in a single ascending‐dose crossover design during three dose‐escalating treatment periods (treatment periods 1‐3). Each participant received a maximum of two single ascending oral doses of GSK3358699 (1, 3, 10, 20, 40 or 30 mg) and one dose of placebo. In treatment period 4, participants were treated with 25 mg of GSK3358699 or placebo followed by an in vivo LPS (cohort 1) or GM‐CSF challenge (cohort 2). Part C of the study was planned to be a repeat‐dose design in sequential cohorts with participants randomized to either 10 mg of GSK3358699 or placebo daily for 14 days. However, the study was terminated during cohort 5 and cohorts 6‐8 were not conducted. GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; LPS, lipopolysaccharide; PBO, placebo; PD, pharmacodynamic; PK, pharmacokinetic
