FIG. 2.
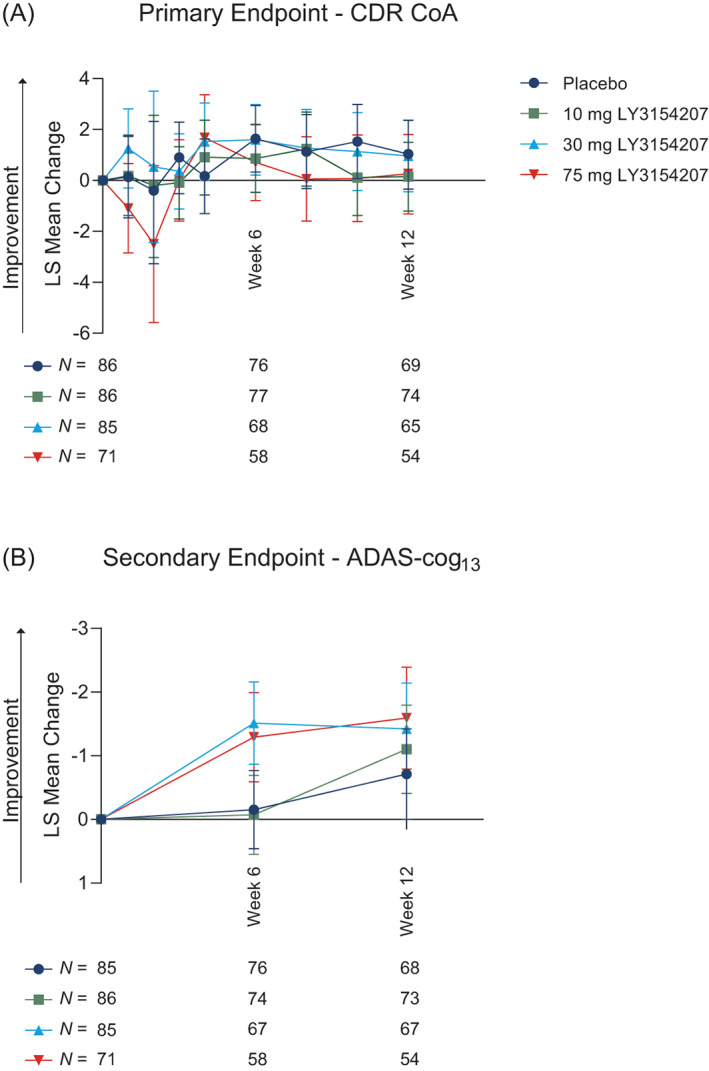
Cognitive outcomes using Cognitive Drug Research‐Continuity of Attention (CDR‐CoA) and Alzheimer's Disease Assessment Scale‐Cognitive Subscale 13 (ADAS‐cog13). CDR‐CoA (A) was the primary endpoint, while ADAS‐cog13 (B) was a key secondary endpoint in the PRESENCE trial. P values between treatment groups were non‐significant and are not shown. N numbers per group are shown below the associated time point. Abbreviations: LS, least squares; N, number of participants.
