Abstract
Aims
Newborn mice and humans display transient cardiac regenerative potential that rapidly declines postnatally. Patients who survive a myocardial infarction (MI) often develop chronic heart failure due to the heart’s poor regeneration capacity. We hypothesized that the cardiac ‘regenerative-to-scarring’ transition might be driven by the perinatal shifts observed in the circulating T-cell compartment.
Methods and results
Post-MI immune responses were characterized in 1- (P1) vs. 7-day-old (P7) mice subjected to left anterior descending artery ligation. Myocardial infarction induced robust early inflammatory responses (36 h post-MI) in both age groups, but neonatal hearts exhibited rapid resolution of inflammation and full functional recovery. The perinatal loss of myocardial regenerative capacity was paralleled by a baseline increase in αβ-T cell (CD4+ and CD8+) numbers. Strikingly, P1-infarcted mice reconstituted with adult T-cells shifted to an adult-like healing phenotype, marked by irreversible cardiac functional impairment and increased fibrosis. Infarcted neonatal mice harbouring adult T-cells also had more monocyte-derived macrophage recruitment, as typically seen in adults. At the transcriptome level, infarcted P1 hearts that received isolated adult T-cells showed enriched gene sets linked to fibrosis, inflammation, and interferon-gamma (IFN-γ) signalling. In contrast, newborn mice that received isolated Ifng –/– adult T-cells prior to MI displayed a regenerative phenotype that resembled that of its age-matched untreated controls.
Conclusion
Physiological T-cell development or adoptive transfer of adult IFN-γ-producing T-cells into neonates contributed to impaired cardiac regeneration and promoted irreversible structural and functional cardiac damage. These findings reveal a trade-off between myocardial regenerative potential and the development of T-cell competence.
Keywords: Cardiac regeneration, Immune system, Myocardial infarction, T-cells, Wound healing
Structured Graphical Abstract
Structured Graphical Abstract.
Schematic representation of T-cell involvement in the neonatal cardiac ‘regenerative-to-scarring’ transition. Cardiac regeneration following myocardial infarction in lymphophenic neonatal mice (upper panel). Adoptive adult T-cell transfer followed by myocardial infarction induced a sustained inflammatory response mainly via interferon-γ and monocyte derived macrophages (CCR2+) leading to impaired neonatal cardiac regeneration (lower panel).
See the editorial comment for this article ‘T cells: a ‘hidden corner’ to be explored for treating heart failure’, by Yike Zhu et al., https://doi.org/10.1093/eurheartj/ehac241.
Translational Perspective.
Upon myocardial infarction, neonate mammals can mount a regenerative response that results in complete cardiac functional recovery, whereas adults typically build up a collagenous scar that subsequently leads to heart failure. Here we show that adoptively transferring adult T-cells into neonates blunted myocardial regeneration and shifted the reparative phenotype towards an adult-like scarring phenotype in an IFN-γ-dependent fashion. These findings demonstrate that the perinatal loss of myocardial regenerative potential is associated with the emergence of T-cell immunocompetence and sheds light on cellular immune mechanisms that can be eventually exploited to modulate the myocardial repair process.
Introduction
Cardiac regeneration is a central scientific aim worldwide. Whereas the adult heart is known to possess limited regenerative capacity, we and others have demonstrated that complete cardiac regeneration occurs in a neonatal mouse model of myocardial infarction (MI).1–3 These findings have been further confirmed in porcine models.4 Moreover, we have previously reported complete functional cardiac recovery in a case of MI in a human newborn, confirming that these observations in experimental animal models might hold translational relevance for humans.5 However, cardiac regenerative potential rapidly disappears after birth, and 7-day-old mice subjected to experimental MI exhibit fibrotic scarring accompanied by long-lasting functional impairment, as typically seen in adults.2,3
The differences observed in neonatal vs. adult myocardial repair can in part be attributed to cardiac-intrinsic mechanisms, as cardiomyocytes retain residual proliferative capacity shortly after birth.6,7 Nevertheless, recent compelling single-cell RNA-sequencing, transcriptome profiling, leucocyte ablation, and comparative biology studies have revealed that immune responses can critically impact myocardial regeneration and scarring.8–15 Myocardial infarction is typically followed by an overt in situ immune-inflammatory response, marked by neutrophil, monocyte, and lymphocyte influx into the injured cardiac tissue.16,17 Yet the precise contributions defined immune cells make to neonatal cardiac regeneration remain largely elusive.
Immunologically, neonates are unique organisms. The perinatal period is characterized by a series of immune alterations marked by distinct immunosuppression. Newborn mammals show important alterations in T-cell distribution, functionality, and intra-thymic development.18,19 For instance, CD4+ T-cells purified from the human umbilical cord blood are intrinsically biased towards acquiring a regulatory phenotype and fail to produce interleukin-17 (IL-17) upon in vitro stimulation.20,21 In adults, T-cell-derived IL-17 has been implicated in adverse chronic tissue remodelling.22 Neonatal T-cells also have blunted capacity to secrete pro-inflammatory mediators such as interferon-gamma (IFN-γ) or tumour necrosis factor (TNF),18 both of which have been linked to post-MI inflammation and fibrosis in adults. These T-cell-derived cytokines critically impact myelopoiesis and the differentiation of monocytes, macrophages, and fibroblasts, other important cellular players in post-MI repair.23–25 Newborn rodents and humans display transient thymic involution, with sharply decreased thymic output and peripheral T-cell lymphopenia.19,26 In mice, the temporal dynamics of thymic T-cell development typically occur in two discontinuous waves. T-cells initially develop on embryonal Days 13–18, and a second wave of T-cell production occurs on postnatal Days 3–6.26 Notably, this latter event temporally coincides with a loss of cardiac regenerative capacity.2
Based on these observations, we hypothesized that the developing T-cell compartment could fuel the myocardial ‘regenerative-to-scarring transition’ that occurs shortly after birth. We therefore characterized and compared the post-MI immune response in postnatal Day 1 (P1, regenerative) and 7-day-old (P7, scarring) mice subjected to left anterior descending artery (LAD) ligation or SHAM control surgery, as well as in neonate mice transplanted with adult T-cells.
Methods
Animals
Animal experiments were approved by the animal ethics advisory board of the Medical University of Innsbruck and the Institute of Molecular Biotechnology of the Austrian Academy of Sciences and authorized by the Austrian Federal Ministry of Education, Science, and Research. Animal experiments conformed to Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Animal experiments were documented according to the Animal Research: Reporting in Vivo Experiments guidelines. Mice were housed in controlled environmental conditions, with room temperature ranging between 22 and 24°C on a 12 h light/dark cycle and provided with standard food and water ad libitum.
Experimental myocardial infarction
Experimental MI was induced by ligation of the left anterior descending artery in postnatal Day 1 or 7 mice, termed P1 and P7, respectively. Endpoint analyses, including flow cytometry, histology, and gene expression assays, were conducted at 1, 4, 7, and 21 days post-injury (dpi). The surgeries were performed by an experienced researcher as previously established by our team27 (more details in Supplementary material online, Methods).
Echocardiography
Cardiac function was determined by two-dimensional transthoracic echocardiography 1 day after injury and then weekly until hearts were harvested (more details in Supplementary material online, Methods).
T-cell purification and adoptive transfer
Bulk T-cells were purified using magnetic cell sorting of untouched CD3+ T-cells freshly isolated from the spleens and lymph nodes of adult female Thy1.1+ or IFN-γ knockout (Ifng –/–) donor mice (12–20 weeks old). After purification, the adult CD3+ T-cells were adoptively transferred into syngeneic P1 neonates 24 h before LAD or SHAM procedure. A detailed description is presented in Supplementary material online, Methods.
IFN-γ-neutralizing antibody
IFN-γ-neutralizing antibody was injected into 4-day-old mice. The injection was repeated after LAD ligation at P7 and 2 dpi. A detailed description is presented in Supplementary material online, Methods.
Flow cytometry analysis
Spleen and collagenase-digested heart samples were assessed by flow cytometry as described.28 Flow cytometry measurements were performed using the CytoFlex (Beckman Coulter) and Attune NxT (ThermoFisher) and further analysed using the FlowJo X (FlowJo LLC Ashland, OR, USA). Compensation for spectral overlap was based on single staining controls and flow cytometry gates were based on unstained control samples. A detailed description is presented in Supplementary material online, Methods.
Histology
For histology, Masson’s trichrome staining, Tunel labelling, and light-sheet fluorescence microscopy were performed as described in Supplementary material online, Methods.
RNA-sequencing and bioinformatics
Left cardiac ventricles were collected from neonatal hearts either left untreated or reconstituted with adult T-cells 36 h and 7 d after myocardial infarction, and RNA was purified using the RNAeasy Plus Micro Kit (Qiagen, Düsseldorf, Germany). Bulk RNA-sequencing, data normalization, and differential expression analysis were performed by the Core Unit Systemmedizin (SysMed) at the University Hospital Würzburg, as described in the Supplementary material online, Methods.
Data analysis
For statistical analysis, the GraphPad Prism Version 8 (GraphPad Software, Inc., La Jolla, CA, USA) was used. Two groups were compared by unpaired Student’s t-test. To compare more than two groups, a two-way ANOVA with Tukey's or Bonferroni's multiple comparisons test was performed. A P-value <0.05 was considered statistically significant. For all experiments with statistical analysis, exact n values are included in the figure legends or within the figure itself. Data are expressed as mean ± standard deviation (S.D.). Echocardiographic analysis, animal measurements, and fibrosis quantification were conducted by one investigator blinded to the experimental procedure. Flow cytometry data were analysed using the FlowJo.
Results
Loss of perinatal regenerative potential is paralleled by shifts in the developing T-cell compartment
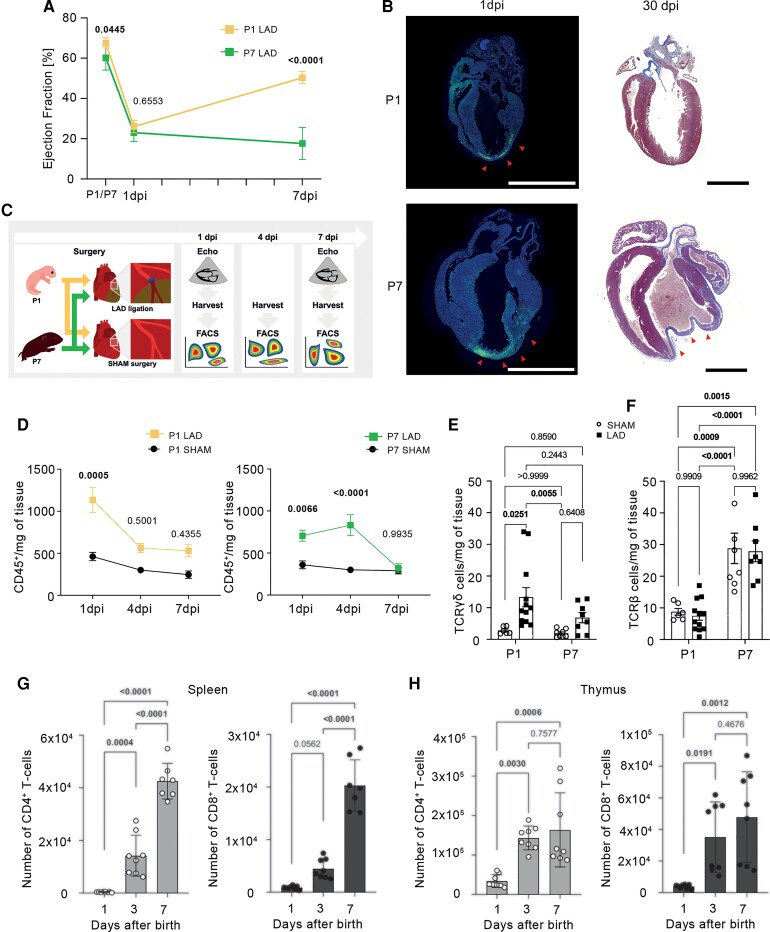
First, we characterized the post-MI healing and adaptive immune responses in P1 vs. P7 mice subjected to LAD ligation. As shown in Figure 1A, infarcted newborn mice (P1) exhibited only transient cardiac functional impairment marked by sharply decreased ejection fraction, followed by rapid and complete functional recovery. In contrast, hearts infarcted at P7 showed sustained functional impairment and fibrotic repair (Figure 1A and B), as previously shown.27 Based on these contrasting cardiac phenotypes observed in P1 and P7, we henceforth term these age groups ‘regenerative’ and ‘scarring-prone’, respectively.
Figure 1.
Loss of perinatal regenerative potential is paralleled by shifts in the developing T-cell compartment. (A) Time course echocardiographic analysis of ejection fraction in different age groups, with left anterior descending artery (LAD) ligation either on postnatal Day 1 (P1, n = 6) or postnatal Day 7 (P7, n = 6). (B) Representative long-axis histological sections from hearts harvested 1 dpi (left panels, tunel-labelled areas evidencing cell death) and 30 dpi (right panels, Masson’s trichrome staining) as reported in27. The arrowheads indicate the cell death area/myocardial scar. Scale bars indicate 2 mm. (C) Graphical representation of the experimental setup. (D) Number of myocardial CD45+ leucocytes per mg of heart tissue. P1 LAD n = 6–12, P1 SHAM n = 4–6, P7 LAD n = 6–10, P7 SHAM n = 6–11. Number of cardiac TCRγδ (E) and TCRβ (F) T-cells per mg of tissue 1 dpi in mice, which underwent LAD ligation/SHAM-surgery at postnatal Day 1 (P1) or postnatal Day 7 (P7), respectively. (G and H) Kinetics of CD4+ and CD8+ T-cells counts in spleen (G) and thymus (H) harvested from 1-, 3-, and 7-day-old mice. P, postnatal day; dpi, days post-injury. Data are expressed as mean values ± S.D. Statistical tests: two-way ANOVA followed by Tukey’s multiple comparisons test (D–F) and one-way ANOVA followed by Tukey’s multiple comparisons test (G and H).
Next, we employed flow cytometry analyses of digested hearts harvested 1, 4, and 7 dpi to characterize the infiltrating immune cells (Figure 1C, Supplementary material online, Figure S1). As shown in Figure 1D, we observed a marked early myocardial influx of CD45+ leucocytes at 1 dpi in both infarcted age groups. No significant inflammatory infiltrate was detected in SHAM-operated hearts. However, while regenerative P1 mice rapidly cleared this inflammatory infiltrate by 4 dpi, the scarring-prone P7 animals showed a more persistent local inflammatory response. This age-dependent time course of myocardial inflammation mirrored cardiac functional outcomes as assessed by echocardiography (Figure 1A).
Flow cytometry analyses of collagenase-digested heart samples further revealed important shifts in the T-cell compartment across the age groups. Regenerative P1 mice subjected to MI exhibited an early myocardial influx of γδ-T-cells (CD45+CD11b−TCRγδ+), whereas scarring-prone P7 mice showed increased αβ-T-cells (CD45+CD11b−TCRβ+, comprising the CD4+ and CD8+ subsets) during both steady-state and post-MI conditions (Figure 1E and F). To better understand the higher counts of myocardial T-cells found in P7 mice at baseline, we also characterized systemic T-cell distribution in naïve P1–P7 mice. Strikingly, we found that splenic CD4+ and CD8+ T-cells were almost absent at birth but rapidly expanded over the first postnatal week, peaking on Day 7 (Figure 1G). Similar kinetics were observed for mature CD4+ and CD8+ single-positive T-cell development in postnatal thymi (Figure 1H). Taken together, these data show that murine cardiac regenerative potential is limited to the first few postnatal days, and its decline is paralleled by rising numbers of CD4+ and CD8+ T-cells in steady-state conditions, both locally and systemically.
Adult T-cells switch neonatal cardiac regeneration to tissue scarring
After identifying important age-related shifts in the systemic distribution of CD4+ and CD8+ T-cells during the first postnatal week, we hypothesized that the T-cells arising systemically between P1 and P7 could provide an additional layer of post-MI inflammation by expressing distinct soluble mediators that are not present in lymphopenic regenerating neonates. Thus, to assess a possible causal relationship between postnatal loss of myocardial regenerative potential and the age-related rise in systemic T-cell counts, we adoptively transferred untouched CD3+ T-cells purified from adult Thy1.1+ wildtype (WT) mice into neonatal (P1) Thy1.2+ WT recipients prior to LAD ligation (Figure 2A). Phosphate-buffered saline (PBS)-injected infarcted animals were taken as age-matched controls, while SHAM-operated mice served as controls for the thoracotomy. Flow cytometry confirmed successful T-cell engraftment based on the presence of Thy1.1+ T-cells in the spleens of Thy1.2+ recipients (Figure 2B). We found that conventional T helper cells (CD4+ Foxp3−) accounted for ∼75% of all engrafted cells, whereas CD8+ T-cells accounted for ∼25%. CD4+Foxp3+ regulatory T-cells accounted for <1% of engrafted cells (Figure 2C). This heterochronic cell transfer model generated chimeric mice bearing both a neonatal cardiovascular system and an adult-like immune cell compartment, thereby providing a unique opportunity to decompose the individual contribution of each compartment to the regeneration-to-scarring transition.
Figure 2.
Adult T-cell transfer impairs neonatal cardiac regeneration. (A) Graphical representation of the experimental setup. Adult Thy1.1+ T-cells were transferred into Thy1.2+ neonatal (P1) WT recipients. (B) The successful transfer of Thy1.1+ cells was confirmed by flow cytometry analysis of the spleen. (C) Characterization of transferred Thy1.1+ cell subpopulation in the spleen. (D) Time course echocardiographic analysis of ejection fraction. SHAM control and SHAM + Adult T-cells n = 6, left anterior descending artery (LAD) control n = 8, LAD + adult T-cells n = 9. (E) Representative images of 2D echocardiogram in the left parasternal long-axis view. (F) Representative serial long-axis histological sections from hearts harvested 21 dpi, stained with Masson’s trichrome. The arrowheads indicate the fibrotic area. Original magnification 20×, scale bar 1000 µm (F, upper), 40×, scale bar 100 µm (F, lower). (G) Quantification of the fibrosis percentage in the left ventricle 21 dpi. P, postnatal day; dpi, days post-injury. Data are expressed as mean values ±S.D. (B, D, G). Statistical test: two-way ANOVA followed by Tukey’s multiple comparisons test (B, D, G).
The cardiac functionality in control vs. T-cell-transferred groups was monitored by echocardiography for 21 days following MI. One day after LAD ligation (1 dpi), we observed significantly decreased left ventricular systolic function in both control and T-cell-transferred groups, thus confirming successful MI induction (Figure 2D and E). Nevertheless, whereas the control infarcted neonates rapidly recovered cardiac function, age-matched animals transplanted with adult T-cells exhibited sustained left ventricular impairment, as typically seen in adults (Figure 2D and E). Histological analysis performed at 21 dpi showed collagen-rich scar formation at the ischaemic injury site in the T-cell-reconstituted mice but not in control infarcted neonates (Figure 2F and G). T-cell transplantation alone did not result in any apparent cardiac phenotype in SHAM-operated mice (Figure 2D, F, G). Taken together, these results show P1-infarcted mice transferred with adult T-cells developed an adult-like scarring phenotype, as evidenced by irreversible cardiac functional impairment and increased fibrosis.
Adult T-cells fuel post-myocardial infarction cardiac inflammation and blunt cardiomyocyte development
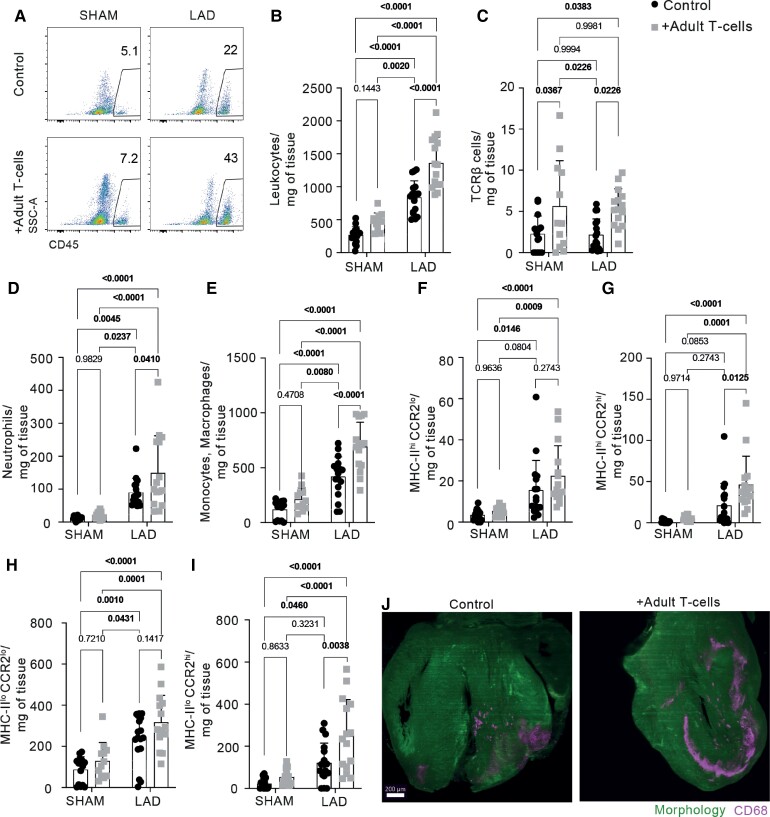
To gain mechanistic insights, we immunophenotyped myocardial immune cells from both infarcted neonates that received isolated adult T-cells and age-matched controls. We harvested the hearts and spleens 36 h after LAD/SHAM surgery and analysed the cell populations by flow cytometry (Figure 3, Supplementary material online, Figure S2). Adult T-cell transplantation into P1 neonate recipients resulted in exacerbated early leucocyte influx into the infarcted myocardium (Figure 3A and B). This shift was mapped to increases in cardiac neutrophils, monocyte-derived macrophages, and T-cells (Figure 3C–E). Further, while tissue-resident macrophages (CCR2loMHC-II+/−) dominated the post-MI responses in control neonates, P1 mice that received adult T-cells exhibited significantly elevated numbers of both cardiac resident macrophages and monocyte-derived macrophages (Figure 3F–I). Three-dimensional reconstructions of P1 LAD-injured hearts by light-sheet fluorescent microscopy further confirmed a marked accumulation of CD68+ macrophages in infarcted hearts from adult T-cell-recipient mice, compared with the PBS-treated controls (Figure 3J). These changes in cardiac cell composition might help explain our cardiac functional observations because while cardiac resident macrophages have been previously linked to tissue regeneration,15 monocyte-derived macrophages have been shown to have a distinct pro-inflammatory transcriptomic signature that fuels adverse remodelling.29 As an additional control, we also transferred adult T-cells into P7 animals that, in contrast to P1, already harbour significant amounts of circulating T-cells. In this experimental condition, the engrafted T-cells (primarily conventional CD4+Foxp3−) also impacted the time course of local immune responses, promoting stronger leucocyte infiltration on Day 4 after MI (see Supplementary material online, Figures S3 and 4). Moreover, the adoptive transfer of adult T-cells into P7 recipients prior to LAD ligation raised mortality in this group (see Supplementary material online, Figure S3G) without impacting other cardiac parameters (see Supplementary material online, Figure S3D and E).
Figure 3.
Post-MI in situ inflammatory response following adult T-cell transplantation. (A) Flow cytometry gating strategy for cardiac samples, harvested 1 dpi. Dynamics of myocardial infiltration of total leucocytes (B) and TCRβ T-cells (C). Dynamics of cardiac neutrophils (D, defined as live CD45+CD11b+Ly6G+) and monocytes/macrophages (E, defined as live CD45+CD11b+Ly6G-CD64+). (F–I) Cardiac macrophage subpopulation characterization according to MHC-II and CCR2 expression. (J) Light-sheet microscopy showing macrophage infiltration (CD68) in control vs. T-cell-transplanted hearts harvested 1 dpi. Scale bar: 200 µm. Data are expressed as mean values ±S.D. Statistical tests: two-way ANOVA followed by Tukey’s multiple comparisons test (B–I).
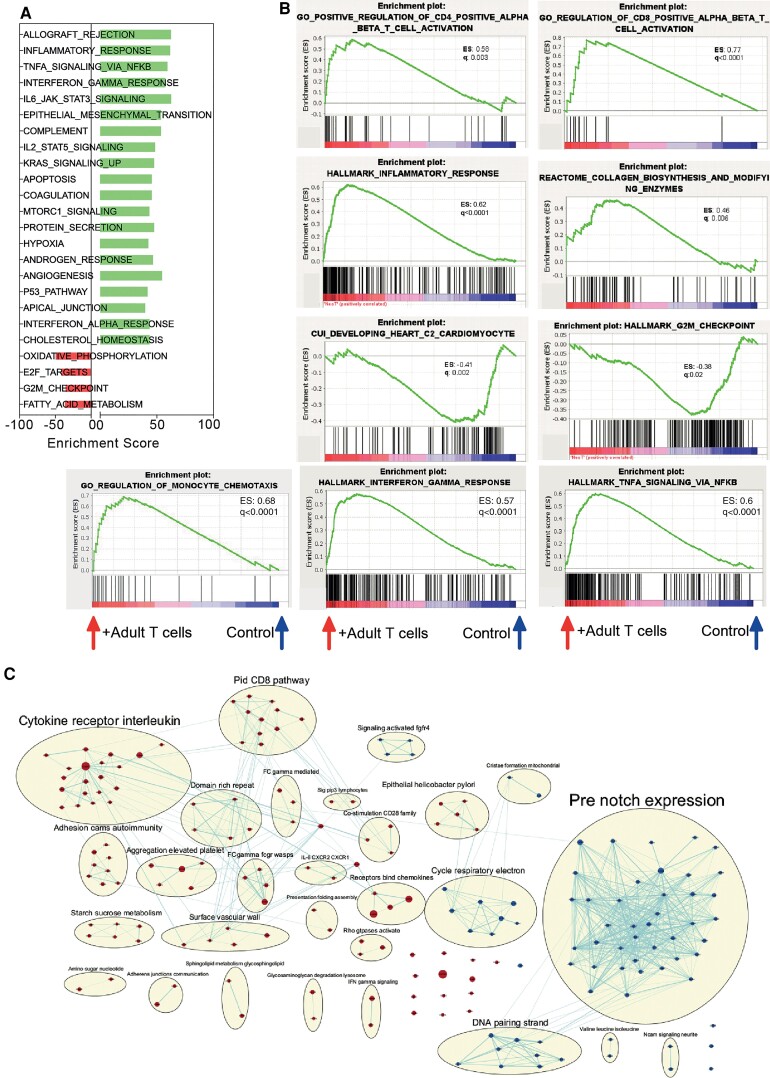
Finally, we performed bulk RNA-seq on myocardial samples obtained at 1 and 7 dpi from both infarcted neonates that received transferred T-cells and age-matched controls. Gene set enrichment analyses revealed that the presence of adult T-cells in the context of neonatal MI (i.e. in otherwise lymphopenic mice) promoted several pathways linked to inflammatory and adaptive immune responses. In hearts that received adult T-cell transfer, we found the following terms: ‘inflammatory response’, ‘TNF signalling via NFκB (nuclear factor kappa B)’, ‘IFN-γ responses’, ‘Jak-STAT3 signalling’, ‘IL-2-STAT5 signalling’, and ‘Hypoxia’ (Figure 4A). Additionally, these T-cell-recipient neonates also had enrichment of gene sets annotated to ‘hallmark IFN-γ responses’, ‘hallmark TNF signalling via NFκB’, and ‘positive regulation of CD4+ and CD8+ T-cell activation’. These findings indicate that the transferred adult T-cells express important soluble mediators, such as IFN-γ, which then contribute to inflammatory signalling in the injured myocardium. Further, we found that gene sets annotated to ‘collagen biosynthesis’ and ‘regulation of monocyte chemotaxis’ were also enriched in infarcted neonates that had received adult T-cell transfer (Figure 4B). These observations are in line with our histological and flow cytometry results, shown in Figures 2 and 3, and provide molecular insights into the functional phenotypes observed. Strikingly, our transcriptome analyses further revealed decreased expression of transcripts related to cardiomyocyte development and mitosis checkpoints in the hearts obtained from infarcted neonates transplanted with adult T-cells (Figure 4B and C, Supplementary material online, Figure S5). Similar RNA-seq analyses performed at 7 dpi indicated that the anti-mitotic effects of adult T-cells in the injured myocardium were long-lasting (see Supplementary material online, Figure S5). Taken together, our data reveal a pronounced cardiac influx of inflammatory monocytes/macrophages and increased TNF and IFN-γ signalling in infarcted neonates that were transplanted with adult T-cells.
Figure 4.
RNA-seq analyses reveal that adult T-cells impact neonatal inflammatory response and proliferative potential. (A) Enrichment score of hallmark gene sets found differentially expressed in neonatal hearts of T-cell-transferred recipients at 1 dpi (false discovery rate < 0.05). (B) Enrichment plots for transcripts related to ‘Positive regulation of CD4 positive alpha beta T-cell activation’, ‘Positive regulation of CD8 positive alpha beta T-cell activation’, ‘Hallmark inflammatory responses’, ‘Reactome collagen biosynthesis’, ‘Developing heart’, ‘G2/M checkpoint’, ‘Regulation of monocyte chemotaxis’, ‘Hallmark interferon-gamma response’ and ‘Hallmark tumour necrosis factor signalling via NFKB’. ES: enrichment score, q: false rate discovery. (C) Network illustrating biological processes and pathways upregulated (red) or downregulated (blue) in hearts from P1 T-cell-transferred mice, harvested 1 dpi.
T-cell-derived IFN-γ mediates the influx of inflammatory monocytes and macrophages after myocardial infarction and contributes to regeneration-to-scarring progression
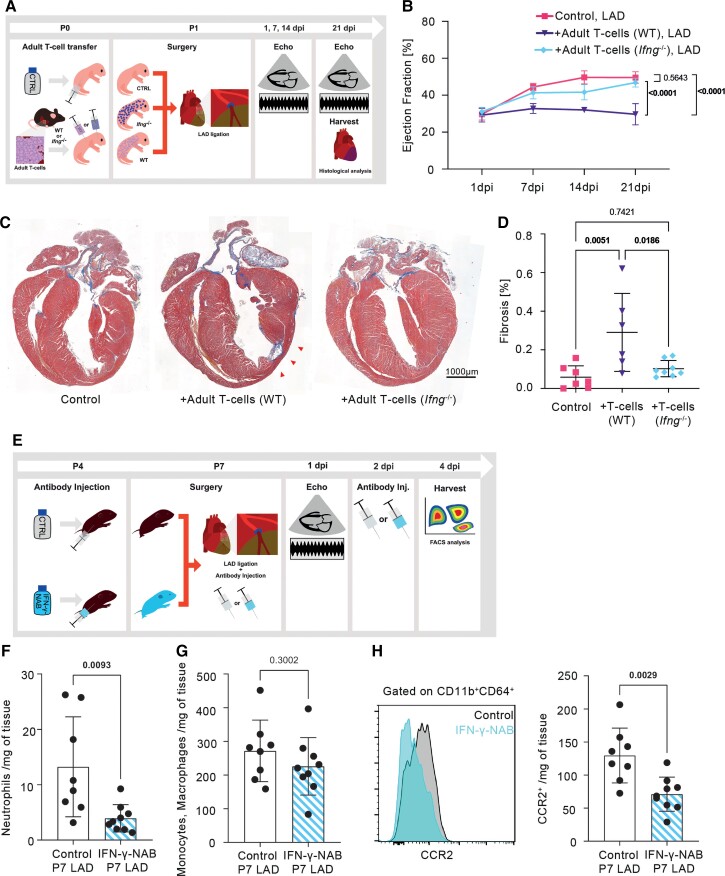
Building on the observations that (i) regenerating neonatal mice are lymphopenic and (ii) promoting an adult-like T-cell compartment in neonates blunted their regeneration capacity, fuelled myocardial IFN-γ signalling and boosted inflammatory monocytes recruitment, and knowing that T-cells are the major paracrine source of IFN-γ,30 we decided to assess whether T-cell-derived IFN-γ contributes to regeneration-to-scarring progression. Therefore, we transplanted T-cells isolated from adult Ifng –/– mice into WT neonatal recipients prior to LAD ligation and monitored their cardiac function and healing outcomes by echocardiography and histology. Neonatal mice with transferred WT adult T-cells and PBS-treated neonatal mice served as controls (Figure 5A). While adoptive transfer of adult T-cells into neonates prior to MI induction again caused the permanent cardiac impairment, IFN-γ loss in the donor cells curtail the detrimental effects of adult T-cell transplantation, and Ifng –/–-transferred recipients exhibited the physiological cardiac regeneration typically seen in neonates (Figure 5B). Histological analyses performed at 21 dpi further confirmed that while WT adult T-cells promoted myocardial fibrosis in infarcted neonates, Ifng –/–-T-cells did not impair tissue regeneration (Figure 5C and D).
Figure 5.
T-cell transfer from adult Ifng –/– mice does not impair neonatal cardiac regeneration. (A) Graphical representation of the experimental setup. Ifng –/– and Thy1.1+ WT T-cells were transferred into Thy1.2+ WT neonatal recipients (P1). (B) Time course echocardiographic analysis of ejection fraction. Left anterior descending artery (LAD) control n = 7, LAD + adult T-cells (WT) n = 6, LAD + adult T-cells (Ifng –/–) n = 8. (C) Representative serial long-axis histological sections from hearts harvested 21 dpi, stained with Masson’s trichrome. The red arrowheads indicate the fibrotic area. Original magnification 20×, scale bar: 1000 µm. (D) Quantification of the fibrosis percentage in the left ventricle 21 dpi. (E) Graphical representation of the experimental setup. Interferon-gamma-neutralizing antibody was injected into 4-day-old mice before LAD ligation as well as twice after ligation and harvested 4 dpi for flow cytometry analysis. (F) Quantification of cardiac neutrophil (defined as live CD45+CD11b+Ly6G+) and (G) monocyte/macrophage (defined as live CD45+CD11b+Ly6G-CD64+) infiltration into infarcted hearts. (H) Cardiac macrophage subpopulation characterization according to CCR2 expression. P, postnatal day; dpi, days post-injury; NAB, neutralizing antibody. Data are expressed as mean values ±S.D. (B, D, F–H). Statistical test: one-way ANOVA followed by Bonferroni’s (B) or Tukey’s (D) multiple comparisons test; unpaired t-test (F–H).
Next, to assess the possible contribution of IFN-γ to the perinatal regeneration-to-scarring shift in a more physiological condition, we analysed the post-MI immune responses of scarring-prone P7 mice treated with either IFN-γ-neutralizing antibody (IFN-γ-NAB) or PBS before and after LAD ligation (Figure 5E). Flow cytometry analyses of hearts harvested at 4 dpi revealed that IFN-γ-NAB treatment reduced myocardial neutrophil infiltration (Figure 5F) and attenuated the infiltration of CCR2hi pro-inflammatory monocyte-derived macrophages without impacting the total monocyte/macrophage cell counts (Figure 5H).
Discussion
Cardiac regeneration has been a challenging scientific goal for more than two decades. Despite the initial promising preclinical success, until now all proposed concepts failed or showed only marginal, non-sustained clinical benefits.31 Recent observations revealing a narrow postnatal window of cardiac regeneration in various mammalian species, including mice, pigs, and humans, have opened novel perspectives to study bona fide myocardial regeneration in mammalian systems.2–5,32 However, the mechanisms underlying the dichotomy between neonatal myocardial regeneration and the scarring typically seen in adults following myocardial injury remain poorly understood. In the present study, we devised a new ‘heterochronic chimera’ approach in which lymphopenic neonate mice were transplanted with a bulk T-cell compartment purified from adult donors. The study of post-MI repair in such neonates harbouring an adult-like adaptive immune system allowed us to unravel, for the first time, the differential contributions of cardiac-intrinsic vs. immune-mediated mechanisms driving the regeneration-to-scarring transition. After performing echocardiographic functional assessments, immunophenotyping, 3D imaging, bulk RNA-sequencing, and targeted genetic ablation approaches in heterochronic chimeras, we found that the presence of a mature IFN-γ-producing T-cell compartment overrides the cardiac-intrinsic regenerative potential seen in neonates and favours fibrotic repair (Structured Graphical Abstract). Since the vast majority of engrafted T-cells were CD4+ Foxp3−, these anti-regenerative effects might be primarily ascribed to the pro-inflammatory activity of conventional T helper cells. Mechanistically, we found that transplanting adult T-cells into neonates was associated with a stronger local inflammatory response, primarily driven by monocyte-derived macrophages (CCR2+) and with stronger myocardial IFN-γ signalling. The causal association between the parallel rise in IFN-γ competence and the loss of regenerative capacity was confirmed by genetic ablation and antibody neutralization. Monocyte-driven post-MI inflammation is typically seen in scarring-prone adult mice but not in neonates,16 whereas the presence of CCR2− embryonic-derived resident macrophages has been linked to cardiac regeneration.15 Therefore, the T-cell- and IFN-γ-mediated shifts in the myeloid cell compartment might also help explain the perinatal loss of cardiac regeneration and the phenotypes observed in this study.
Even though T-cells are not the most abundant leucocyte subset infiltrating infarcted hearts, activated CD4+ and CD8+ T-cells can express a plethora of inflammatory mediators, which can critically impact the activity of all other myocardial subsets, including monocytes/macrophages and cardiac fibroblasts. Interferon-gamma, for instance, is mainly produced by conventional CD4+ (Th1) and CD8+ (cytotoxic) T-cells and has been shown to promote myelopoiesis,23 to induce macrophage activation and polarization towards an inflammatory phenotype24 and to promote cardiac fibrosis in a non-ischaemic model of heart failure in adult mice.33 Likewise, T-cell-derived TNF also promotes fibroblast differentiation.25 These soluble factors can act both systemically and locally, and they ultimately contribute to myocardial inflammation and fibrosis.34 These considerations, supported by our transcriptomic findings and by functional IFN-γ-ablation experiments, might help explain how T-cells can impact the myocardial milieu despite being present at relatively low frequencies in the injured myocardium.
The RNA-sequencing analyses herein presented also revealed that the adoptive transfer of adult T-cells into neonate recipients was associated with an inhibition of pathways related to cardiomyocyte development, cell cycle control, and energy metabolism. In a previous study, Li et al. 35 reported that TNF and IFN-γ can inhibit the proliferation and induce apoptosis of neonatal cardiomyocytes in vitro. Our results provide further in vivo evidence that, besides providing an additional layer of immunoregulation during tissue repair, the developing adaptive immune system may also impact fundamental aspects of cardiomyocyte development and its proliferative potential. In another recent study, Li, et al.36 reported that Foxp3+ regulatory T-cells support neonatal myocardial regeneration and could act as a source of paracrine factors that support cardiomyocyte proliferation. Their findings are interesting, but we think ours stem from distinct mechanisms, because the Foxp3+ regulatory T-cells remained barely detectable in our cell transfer model. In fact, our observations were mainly associated with the predominant engraftment of Foxp3− conventional T helper cells, thereby suggesting they exert rather pro-fibrotic effects that can override intrinsic regenerative potential as the animals develop.
In adult mammals, the pro-fibrotic effects of T-cells have already been documented in different experimental models of MI and pressure overload-induced heart failure.22,28,33,37,38 Nevertheless, to the best of our knowledge, the present study provides the first evidence that the emergence of an IFN-γ-producing αβT-cell compartment is a critical event that constrains and commits myocardial repair towards rapid scarring, at the cost of full regeneration. These observations seem to have deep evolutionary roots, as previous studies on amphibian regeneration have also indicated a trade-off between tissue regenerative potential and the emergence of T-cell competence.14
It is important to stress that the outcome of post-MI T-cell responses and fibrosis is neither detrimental nor salutary per se, as it greatly depends on context and timing. In newborn mammals capable of complete cardiac regeneration, IFN-γ responses by adult conventional Foxp3− CD4+T-cells mediated cardiac fibrosis in detriment of regeneration and contributed to a poorer outcome. However, in adult mammals, antigen-specific Foxp3+ CD4+ T-regs responses can foster reparative fibrosis and thus help preserve the integrity of a post-mitotic organ devoid of cardiac regenerative potential.28 Yet, when exacerbated, the fibrotic repair of adult infarcted hearts also contributes to adverse remodelling that ultimately results in heart failure.33,39
Limitations
Although, we found that adult T-cells are causally linked to the loss of myocardial regenerative potential in neonates, it is important to stress that adult lymphocyte-deficient mice still lack the capacity for myocardial regeneration.40 These findings reinforce that, while T-cells might favour fibrotic repair detrimental to regeneration, neonatal myocardial regeneration itself is a multi-layered process resulting from a dynamic interaction amongst different cardiac-intrinsic and systemic factors, and no single mechanism can account for this complexity. A recent study has reported, though, that specifically ablating CD4+ T-cells could restore myocardial regenerative capacity in P8 mice but not in adults.35
Future directions
These observations indicate that, besides providing additional immunoregulation during tissue repair, the developing adaptive immune system may also impact fundamental aspects of cardiomyocyte biology and its proliferative potential, which should be assessed in future studies. Thus, based on the results presented here, we propose that future studies aiming to promote full cardiac regeneration41 should also consider devising strategies to blunt fibrosis-prone T-cell responses, while translational strategies seeking to support salutary fibrotic repair that could help preserve cardiac structure should focus on methods to boost myocardial T-cell activity. Moreover, considering that T-cells primarily impact myocardial biology via crosstalk with monocytes/macrophages, therapeutic approaches targeting post-MI T-cell responses may have greater impact if combined with complementary protocols addressing monocyte migration.42
From a broader perspective, specifically targeted anti-inflammatory strategies are being currently tested for acute coronary syndromes. Cardiac regeneration and scarring are complicated processes, and our study provides novel important mechanistic insights into how adaptive immune responses foster myocardial fibrosis, while suppressing regeneration. Our data thus support the rationale of the ongoing multi-centre Phase I–II RCT CLEVER-ACS (ClinicalTrials.gov Identifier: NCT01529554) that tests how short-term mTOR inhibition impacts infarct size and thereby determines the clinical efficacy of specific immunosuppression on post-infarct remodelling. Taken together, these lines of inquiry have clinical implications for post-infarct treatment and justify novel regenerative strategies tackling several factors in a time-dependent manner.
Conclusion
By studying neonate mice harbouring an adult T-cell compartment, the findings herein presented shed light on the mechanisms controlling the myocardial regeneration-to-scarring transition and provide novel insights linking the emergence of a mature T-cell compartment with the loss of cardiac regeneration potential.
Supplementary Material
Acknowledgements
We thank the VBCF Histo Pathology Facility for their services. Furthermore, we would like to thank the Cardiac Surgery Research Group (J. Holfeld) and the Experimental Anaesthesia Research Group (J. Martini) in Innsbruck for sharing their facilities. We thank Manfred Kopf (Institute of Molecular Health Sciences, ETH Zurich) for providing the Interferon-gamma knockout mice and Prof. Daniel Mucida for the critical suggestions.
Contributor Information
Theresa Dolejsi, Department of Internal Medicine III (Cardiology and Angiology), Medical University of Innsrbuck, Anichstraße 35, 6020 Innsbruck, Austria.
Murilo Delgobo, Department of Internal Medicine I, University Hospital Würzburg, Oberdürrbacher Straße 6, 97080 Würzburg, Germany; Comprehensive Heart Failure Center, University Hospital Würzburg, Am Schwarzenberg 15, D-97078 Würzburg, Germany.
Thomas Schuetz, Department of Internal Medicine III (Cardiology and Angiology), Medical University of Innsrbuck, Anichstraße 35, 6020 Innsbruck, Austria.
Luigi Tortola, Institute of Molecular Health Sciences, ETH Zurich, Otto-Stern-Weg 7, 8093 Zurich, Switzerland.
Katrin G Heinze, Rudolf Virchow Center, University of Würzburg, Josef-Schneider-Straße 2, 97080 Würzburg, Germany.
Ulrich Hofmann, Department of Internal Medicine I, University Hospital Würzburg, Oberdürrbacher Straße 6, 97080 Würzburg, Germany; Comprehensive Heart Failure Center, University Hospital Würzburg, Am Schwarzenberg 15, D-97078 Würzburg, Germany.
Stefan Frantz, Department of Internal Medicine I, University Hospital Würzburg, Oberdürrbacher Straße 6, 97080 Würzburg, Germany; Comprehensive Heart Failure Center, University Hospital Würzburg, Am Schwarzenberg 15, D-97078 Würzburg, Germany.
Axel Bauer, Department of Internal Medicine III (Cardiology and Angiology), Medical University of Innsrbuck, Anichstraße 35, 6020 Innsbruck, Austria.
Frank Ruschitzka, Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, CH-8091 Zurich, Switzerland.
Josef M Penninger, Institute of Molecular Biotechnology of the Austrian Academy of Sciences, Dr-Bohr-Gasse 3, 1030 Vienna, Austria; Department of Medical Genetics, Life Sciences Institute, University of British Columbia, 2350 Health Sciences Mall, Vancouver, BC, Canada.
Gustavo Campos Ramos, Department of Internal Medicine I, University Hospital Würzburg, Oberdürrbacher Straße 6, 97080 Würzburg, Germany; Comprehensive Heart Failure Center, University Hospital Würzburg, Am Schwarzenberg 15, D-97078 Würzburg, Germany.
Bernhard J Haubner, Department of Internal Medicine III (Cardiology and Angiology), Medical University of Innsrbuck, Anichstraße 35, 6020 Innsbruck, Austria; Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, CH-8091 Zurich, Switzerland.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the Austrian Science Fund (FWF, research grant ERA-CVD JTC2018 INNOVATION, I 4161-B, to B.J.H.), by the intramural funding programme of the Medical University of Innsbruck for young scientists [MUI-START, Project (2017-01-014), to B.J.H.] and Medizinischer Forschungsfonds Tirol (MFF, to B.J.H.). J.M.P. is supported by the Austrian Federal Ministry of Education, Science and Research; the Austrian Academy of Sciences and the City of Vienna; the Austrian Science Fund (FWF) Wittgenstein award (Z 271-B19); the T. von Zastrow foundation and the Canada 150 Research Chairs Program (F18-01336). G.C.R. is supported by the Interdisciplinary Centre for Clinical Research Würzburg (E-354), the German Research Foundation (DFG grant 411619907), the European Research Area Network—Cardiovascular Diseases/German Federal Ministry of Education and Research (ERANET-CVD JCT2018, AIR-MI Consortium, BMBF-grant 01KL1902). U.H. was supported by the German research foundation (DFG grant 391580509). G.C.R., U.H., and S.F. lead projects integrated in the Collaborative Research Centre ‘Cardio-Immune interfaces’, funded by the German Research foundation (SFB1525 grant number 453989101). Prof. Ruschitzka has not received personal payments by pharmaceutical companies or device manufacturers in the last 3 years (remuneration for the time spent in activities, such as participation as steering committee member of clinical trials and member of the Pfizer Research Award selection committee in Switzerland, were made directly to the University of Zurich). The Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research-, educational- and/or travel grants from Abbott, Amgen, Astra Zeneca, Bayer, Berlin Heart, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Corteria, Daiichi, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Kantar, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Roche Diagnostics, Sahajanand IN, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, Trama Solutions, V- Wave, Vascular Medical, Vifor, Wissens Plus, ZOLL. The research and educational grants do not impact on Prof. Ruschitzka`s personal remuneration.
Conflict of interest: none declared.
References
- 1. Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. Dynamics of cell generation and turnover in the human heart. Cell 2015;161:1566–1575. [DOI] [PubMed] [Google Scholar]
- 2. Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, et al. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 2012;4:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science 2011;331:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye L, D'Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, et al. Early regenerative capacity in the porcine heart. Circulation 2018;138:2798–2808. [DOI] [PubMed] [Google Scholar]
- 5. Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ Res 2016;118:216–221. [DOI] [PubMed] [Google Scholar]
- 6. Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laflamme MA, Murry CE. Heart regeneration. Nature 2011;473:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Cui M, Shah AM, Ye W, Tan W, Min YL, et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc Natl Acad Sci U S A 2019;116:18455–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui M, Wang Z, Chen K, Shah AM, Tan W, Duan L, et al. Dynamic transcriptional responses to injury of regenerative and non-regenerative cardiomyocytes revealed by single-nucleus RNA sequencing. Dev Cell 2020;53:102–116.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamowicz M, Morgan CC, Haubner BJ, Noseda M, Collins MJ, Abreu Paiva M, et al. Functionally conserved noncoding regulators of cardiomyocyte proliferation and regeneration in mouse and human. Circ Genom Precis Med 2018;11:e001805. [DOI] [PubMed] [Google Scholar]
- 11. Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019;364:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol 2015;17:627–638. [DOI] [PubMed] [Google Scholar]
- 13. Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, et al. Hypoxia induces heart regeneration in adult mice. Nature 2017;541:222–227. [DOI] [PubMed] [Google Scholar]
- 14. Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: an immunologic perspective. Dev Dyn 2003;226:268–279. [DOI] [PubMed] [Google Scholar]
- 15. Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest 2014;124:1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nunes-Silva V, Frantz S, Ramos GC. Lymphocytes at the heart of wound healing. Adv Exp Med Biol 2017;1003:225–250. [DOI] [PubMed] [Google Scholar]
- 18. Schaub B, Liu J, Schleich I, Höppler S, Sattler C, von Mutius E. Impairment of T helper and T regulatory cell responses at birth. Allergy 2008;63:1438–1447. [DOI] [PubMed] [Google Scholar]
- 19. Varas A, Jiménez E, Sacedón R, Rodríguez-Mahou M, Maroto E, Zapata AG, et al. Analysis of the human neonatal thymus: evidence for a transient thymic involution. J Immunol 2000;164:6260–6267. [DOI] [PubMed] [Google Scholar]
- 20. de Roock S, Stoppelenburg AJ, Scholman R, Hoeks SBEA, Meerding J, Prakken BJ, et al. Defective TH17 development in human neonatal T cells involves reduced RORC2 mRNA content. J Allergy Clin Immunol 2013;132:754–756.e3. [DOI] [PubMed] [Google Scholar]
- 21. Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. ‘Default’ generation of neonatal regulatory T cells. J Immunol 2010;185:71–78. [DOI] [PubMed] [Google Scholar]
- 22. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 2019;139:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matatall KA, Shen CC, Challen GA, King KY. Type II interferon promotes differentiation of myeloid-biased hematopoietic stem cells. Stem Cells 2014;32:3023–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang K, Bachu M, Park SH, Kang K, Bae S, Park-Min KH, et al. IFN-γ selectively suppresses a subset of TLR4-activated genes and enhancers to potentiate macrophage activation. Nat Commun 2019;10:3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prados A, Onder L, Cheng HW, Mörbe U, Lütge M, Gil-Cruz C, et al. Fibroblastic reticular cell lineage convergence in Peyer's patches governs intestinal immunity. Nat Immunol 2021;22:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Penit C, Vasseur F. Cell proliferation and differentiation in the fetal and early postnatal mouse thymus. J Immunol 1989;142:3369–3377. [PubMed] [Google Scholar]
- 27. Haubner BJ, Schuetz T, Penninger JM. A reproducible protocol for neonatal ischemic injury and cardiac regeneration in neonatal mice. Basic Res Cardiol 2016;111:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rieckmann M, Delgobo M, Gaal C, Büchner L, Steinau P, Reshef D, et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J Clin Invest 2019;129:4922–4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018;24:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol 2018;9:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madonna R, Van Laake LW, Davidson SM, Engel FB, Hausenloy DJ, Lecour S, et al. Position paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J 2016;37:1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, et al. Regenerative potential of neonatal porcine hearts. Circulation 2018;138:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, et al. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med 2017;214:3311–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez-Shibayama C, Gil-Cruz C, Cheng HW, Onder L, Printz A, Mörbe U, et al. Fibroblastic reticular cells initiate immune responses in visceral adipose tissues and secure peritoneal immunity. Sci Immunol 2018;3:eaar4539. [DOI] [PubMed] [Google Scholar]
- 35. Li J, Liang C, Yang KY, Huang X, Han MY, Li X, et al. Specific ablation of CD4+T-cells promotes heart regeneration in juvenile mice. Theranostics 2020;10:8018–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Yang KY, Tam RCY, Chan VW, Lan HY, Hori S, et al. Regulatory T-cells regulate neonatal heart regeneration by potentiating cardiomyocyte proliferation in a paracrine manner. Theranostics 2019;9:4324–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014;115:55–67. [DOI] [PubMed] [Google Scholar]
- 38. Martini E, Kunderfranco P, Peano C, Carullo P, Cremonesi M, Schorn T, et al. Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation 2019;140:2089–2107. [DOI] [PubMed] [Google Scholar]
- 39. Kallikourdis M, Martini E, Carullo P, Sardi C, Roselli G, Greco CM, et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun 2017;8:14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 2006;114:2056–2064. [DOI] [PubMed] [Google Scholar]
- 41. Hashimoto H, Olson EN, Bassel-Duby R. Therapeutic approaches for cardiac regeneration and repair. Nat Rev Cardiol 2018;15:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J, Seo MJ, Deci MB, Weil BR, Canty JM, Nguyen J. Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. Int J Nanomed 2018;13:6441–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.