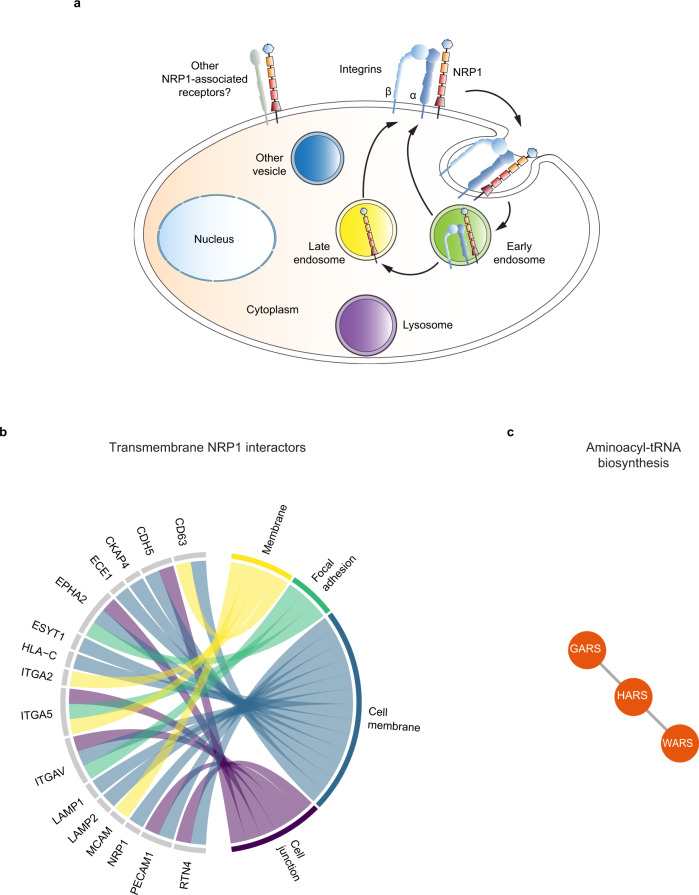
Fig. 2. NRP1 interactome.
a Schematic drawing summarizing the role of NRP1 as a pro-endocytic receptor20,22,23,33 that promotes the internalization and recycling of associated plasma membrane receptors, such as integrins20,33 and other still to be identified receptors (in gray). b Cell adhesion receptors as preferential potential transmembrane NRP1 interactors. Chord diagram of transmembrane proteins found to interact with NRP1. Each protein was assigned to one or more subcellular locations based on UniProt annotation. In addition, to associate with transmembrane proteins mediating or regulating EC adhesion, NRP1 also associated with the endoplasmic reticulum reticulon 4 (RTN4) transmembrane protein that promotes cell adhesion by regulating integrin traffic101. Moreover, our identification of the transmembrane metallopeptidase endothelin converting enzyme-1 (ECE1) as a NRP1 partner on the EC surface may suggest that the TBX1-independent control of cardiac OFT morphogenesis by endothelial NRP1102 may involve ECE1103,104, which functions by degrading receptor-bound extracellular ligands upon endocytosis (Supplementary Data 1). c Aminoacyl-tRNA proteins were found to be significantly enriched in the NRP1 interactome, after excluding transmembrane (b) and endosomal (Supplementary Fig. 2a) NRP1 interactors. STRING was used to perform enrichment analysis of KEGG pathways and to determine the physical and functional protein-protein interactions that define the edges between nodes. The plots was generated with R. Source data are provided as a Supplementary Data file.