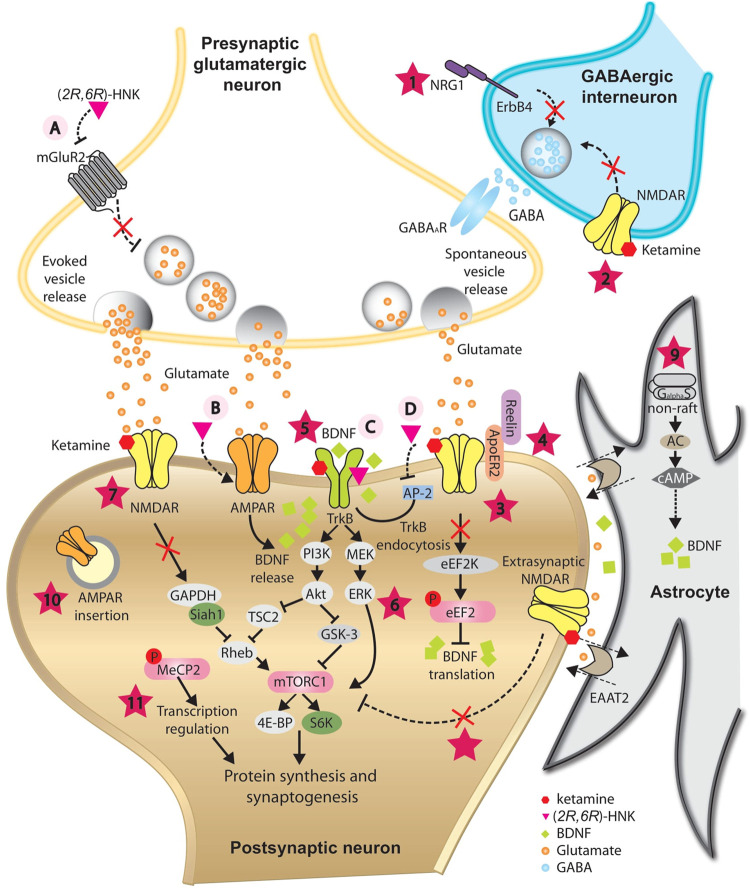
FIGURE 2.
Proposed mechanisms underlying the antidepressant-like effects of ketamine and its metabolite (2R,6R)-HNK. In GABAergic inhibitory interneurons, ketamine is proposed to decrease GABA release onto glutamatergic presynaptic neurons by antagonizing NRG1/ErbB4 signaling pathway (1) and/or blockade of NMDARs (2), resulting in disinhibition of pyramidal neurons. Augmented glutamate transmission activates AMPARs and subsequently enhances BDNF release. In postsynaptic neurons, ketamine is proposed to augment BDNF/TrkB pathway by releasing the inhibition of BDNF translation from eEF2K (3), directly binding with its receptor TrkB (5), and/or by activating the PI3K-Akt and MEK-ERK signaling pathways (6). One of the downstream targets activated via PI3K-Akt and MEK-ERK signaling pathways is mTORC1, which is implicated in synaptogenesis- and synaptic plasticity-relevant processes. Besides direct activation by BDNF/TrkB signaling, blockade of NMDARs by ketamine is proposed to activate mTORC1 indirectly through compromising the ubiquitin-mediated degradation of mTORC1 activator Rheb (7), or by inhibition of extrasynaptic NMDARs (8). At rest, spontaneous vesicle release may play an important role in maintaining the activation of NMDARs at basal level. Reelin/ApoER2 may be implicated in this process and be modulated by ketamine (4). Inhibition of NMDAR by ketamine blockade may promote AMPAR insertion at postsynaptic sites as a homeostatic process (10). BDNF/TrkB signaling pathway may trigger phosphorylation of MeCP2, which is essential for sustained antidepression-like action of ketamine (11). In astrocyte, ketamine is proposed to trigger the translocation of Gas to non-raft domains, leading to enhanced AC activity and elevated BDNF expression (9). (2R,6R)-HNK may act on the presynaptic terminal to increase glutamate release, possibly via inhibition of mGluR2 receptors (A). Elevated glutamate release may activate postsynaptic AMPARs and enhance BDNF release (B). (2R,6R)-HNK may also facilitate the BDNF/TrkB signaling pathway by directly binding with TrkB (C), or by disrupting the interaction between TrkB and AP-2, thus inhibiting the endocytosis of TrkB (D). These mechanisms may have different temporal spatial features and may function synergistically or complementarily, leading to a rebalanced excitatory/inhibitory neurotransmission and restored integrity of neural circuits, which is indispensible for the antidepressant-like action of ketamine and (2R,6R)-HNK.