Figure 5.
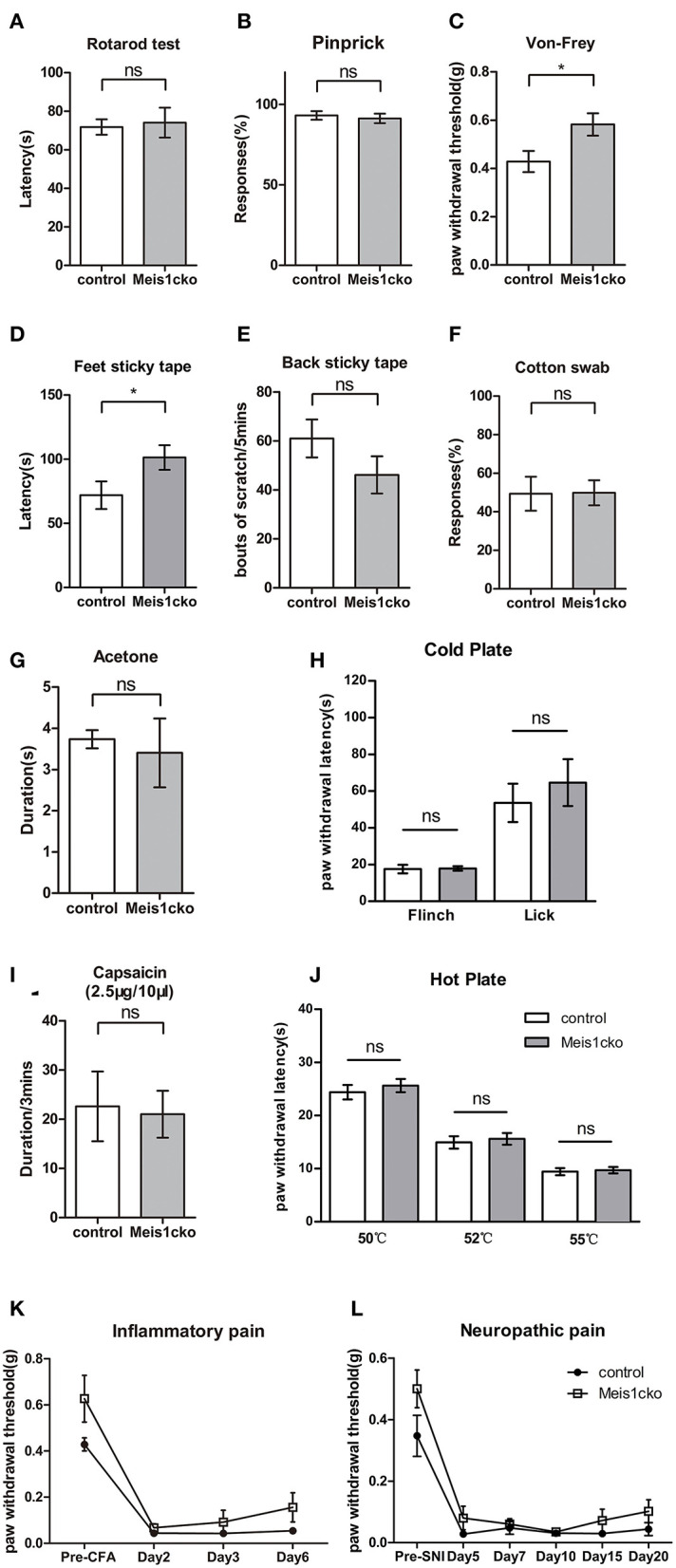
Attenuated response to static touch and light mechanical pain in Meis1cko mice. (A) The rotarod assay. Control and Meis1cko mice showed identical latencies to fall off the rotarod (n = 5 control mice, n = 4 Meis1cko mice; p > 0.05). (B) Acute mechanical pain assessed using the pinprick test. Control and Meis1cko mice showed no significant difference (n = 8 mice per group; p > 0.05). (C) Von Frey assay. Meis1cko mice showed a significantly higher withdrawal threshold than control littermates, and the threshold was increased from 0.43 ± 0.044 g in control mice to 0.58 ± 0.046 g in Meis1cko mice (n = 12 mice per group; p = 0.0187). (D) The latency to remove the sticky tape on the hindpaw was 72 ± 11 s for control mice and 101 ± 10 s for Meis1cko mice (n = 12 mice per group; p = 0.0445). (E) Number of scratching bouts to remove adhesive tape on nape hairy skin (61 ± 8 for control mice vs. 46 ± 8 for Meis1cko mice, n = 10 mice per group; p = 0.187). (F) Percent response to light stroking with a cotton swab (n = 12 mice per group; p > 0.05). (G) Cooling sensation tested by hindpaw exposure to acetone (n = 9 control mice, n = 8 Meis1cko mice; p > 0.05). (H) The latency of forepaw flinching or hindpaw licking on a 0°C cold plate (n = 10 control mice, n = 6 Meis1cko mice; p > 0.05). (I) The time that mice spent licking and flinching after the intraplantar capsaicin injection (n = 5 mice per group; p > 0.05). (J) Hot plate assay. No statistically significant difference was observed between control and Meis1cko mice at 50, 52, and 55°C (n = 12 mice per group, p > 0.05). (K) Meis1cko mice showed a normal response to CFA-induced inflammatory pain (two-way repeated ANOVA, n = 7 mice per group; p > 0.05). (L) Meis1cko mice showed a normal response to SNI-induced neuropathic pain (two-way repeated ANOVA, n = 6 control mice, n = 8 Meis1cko mice; p > 0.05). Error bars represent SEM. (A–J) Significant differences were determined using unpaired Student's t-test: *p < 0.05 ns, Not significant.
