Abstract
Objectives: Malvidin, a dietary anthocyanin can be a potent drug for the treatment of neuronal toxicity. The investigation was aimed to study the antioxidant role of malvidin against aluminum chloride (AlCl3)-induced neurotoxicity in rats. Methods: To evaluate the neuroprotective role of malvidin, the rats were divided into four different groups: group I received saline, group II received AlCl3, and groups III and IV were administered with 100 and 200 mg/kg malvidin after AlCl3 for 60 days. During the evaluation period, all the groups were subjected to a behavioral test. On the 61st day of the study, rat brains were removed and used for a neurochemical assay. Results: From the present study, malvidin ameliorated the effects of AlCl3 on behavioral parameters. Biochemical investigation revealed that oral treatment of malvidin shows neuroprotective effects through regulation of antioxidant levels and neuroinflammation in the AlCl3-exposed rats. Conclusion: The results indicate that malvidin possesses antioxidant activity via acetylcholinesterase inhibition and regulation of oxidative stress in neuronal cells. Hence, malvidin could be a potential drug in correcting Alzheimer’s disease.
1. Introduction
The production house of reactive oxygen species (ROS) is the mitochondria, which regulates the oxidative metabolism and external factors/chemicals in the ATP generation process. The imbalance of ROS and antioxidant enzymes may interfere with the functions of biomolecules (DNA, amino acids, and RNA) causing oxidative stress, which is a key contributor to neurodegeneration.1,2 Aluminum damages the signaling cascades of the oxidative pathway by directly mutating the genes, causing neurotoxicity and ultimately cell death.3−5 Several animal studies showed that chronic exposure to aluminum changes the level of key regulatory hormones of the brain region and stimulates oxidative stress by inhibiting the antioxidant parameters such as catalase (CAT), elevating stress and superoxide dismutase (SOD).6−8 The oxidative stress generated is associated with causing neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and so forth.9 Antioxidant therapy/drugs can be a better option for regulating the antioxidant enzymes and reducing the oxidative burden.
Aluminum (Al) is the third nonrenewable metallic element covering 8% of the earth’s crust, which is detected mainly in natural waters, plants, and animal tissues.10,11 The exposure to aluminum in humans is much more due to its applicability in daily life such as water treatment, paper making, utensil coating, food additives, the color industry, cosmetics, fillers, and pharmaceuticals (drugs such as antacids, antidiarrheal, injections, and vaccines).12,13 Chronic exposure may cause aluminum poisoning, affecting the biological structure and tissues of both animals and human body. Aluminum administration leads to the progression of neurodegenerative diseases such as Alzheimer’s disease, dementia, Parkinson’s disease, and encephalopathy, resulting in functional modification of the brain tissue, cognitive and cholinergic impairment, and neuronal apoptosis.14−16
Chronic exposure to aluminum may affect the signaling pathway of brain neurotransmitters, thus impairing the cognitive function, interfering with the signal transmission to neurons, and causing neuronal damage. Aluminum alters the cholinergic and noradrenergic transmission to neurons through the blood brain barrier. At the molecular level, aluminum has ability to impede the glutamate-NO-cGMP pathway, which may produce behavioral deficits and impaired motor performance in rodents. These findings displayed the role of aluminum in causing deleterious neuronal effects.17,18
Polyphenols includes flavonoids, which are potent antioxidants widely distributed in plants. Anthocyanins also belong to the flavonoid class and show identical properties such as antioxidant, anti-inflammatory, immunomodulatory, and so forth.19,20
Malvidin is an anthocyanin obtained from red wine that is proven to protect against oxidative neuronal damage in both animal cell line and in vivo models. It is known for its antioxidant properties and is used in the treatment of many ailments such as cardiovascular disorders, inflammatory conditions, cancer, and oxidative stress. Malvidin works by targeting the mitogen-activated protein kinase and nuclear kappa B (NFκB) pathway contributing to the anti-inflammatory, antioxidant, and antiapoptotic actions of malvidin.21 An in vivo study showed that malvidin prevented ethanol-induced gastric ulcers by modulating the EGF and COX-1 gene expression.22 A study revealed that malvidin displayed controlled blood pressure action through blocking the angiotensin-converting enzyme I (ACE), which could be beneficial for use as a prophylactic against hypertension.23,24 Another study proved the cardioprotective activity of malvidin in vivo in isoproterenol-induced myocardial infarction in rats.25 Other investigations showed a beneficial effect of malvidin glucosides in skeleton muscle damage in vitro due to its fee radical scavenging property.26 An in vivo study of malvidin on carbon tetrachloride-induced stress showed improved antioxidant parameters.27 Anthocyanins modulate immune-stimulating genes by producing positive effects on CAT, glutathione reductase, and SOD and thereby scavenge the free radicals generated by oxidative stress.28 The current study was conducted to prove the therapeutic and antioxidant potential of malvidin on AlCl3-induced neurotoxicity.
2. Material and Methods
2.1. Chemicals
Malvidin (malvidin-3-glucoside chloride) was purchased from Sigma-Aldrich, USA (≥95.0% purity, CAS no: 643-86-5). Analytical-grade reagents and chemicals were used in the experiment. The commercially available (Modern Lab, M.S. India) biochemical estimation kits for IL-6, IL-1β, and TNF-α were used in this study.
2.2. Experimental Animals
Adult male Wistar rats weighing 200 ± 20 g were kept in standard propylene cages with free access to pellet food and ad libitum water. The rats were grouped and housed at standard room temperature (20 ± 2 °C), and a normal day–night cycle was maintained. All animal experimentation was carried out after receiving protocol (IAEC/BRNCOP/003) approval from the Institutional Animal Ethics Committee.
2.3. Study Plan
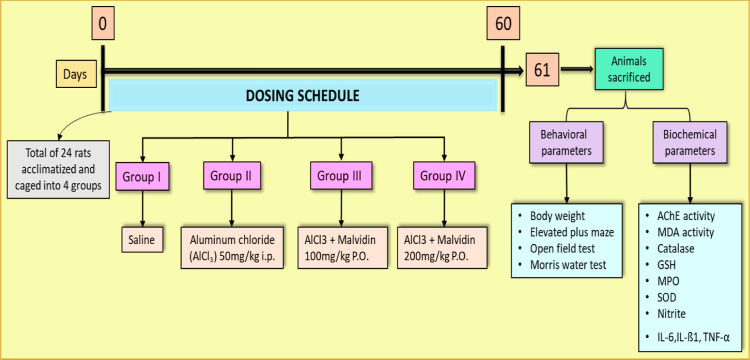
A total of 24 rats were acclimatized to the external atmosphere for at least 7 days and were separated into four groups (six rats per group) (Figure 1).
Figure 1.
Experimental study protocol.
Group I (normal): each rat received a vehicle of 0.5 mL of saline (0.9% NaCl) throughout the study.
Group II (disease control): the rats in this group received AlCl3 [50 mg/kg b wt/day, intraperitoneally (i.p.)] for 60 days.29,30 The animals were observed for changes in food intake, body weight, and physical parameters.
Group III& IV: the animals were treated with aluminum chloride (50 mg/kg) i.p. and subsequently received treatment with malvidin (100 and 200 mg/kg P.O., respectively) 1 h prior to AlCl3 injection for 60 days.
The doses of malvidin were selected to be the same as those from the previous studies.25,31 Behavioral parameters were performed before and after drug administration. After 60 days of treatment with ursolic acid, the rats were sacrificed under high anesthesia. The brains were collected, washed, and preserved in formalin for performing the biochemical assay.
2.4. Acute Toxicological Studies
The study followed the OECD guideline acute toxicity study no. 425 (up and down procedure). The test drug (malvidin) was administered up to the limit dose of 2000 mg/kg in male rats. The animals were observed for the next 14 days for signs of toxicity. Clinical patterns including behavioral changes and changes on the skin and fur, eyes, and body weight were noted.
2.5. Physical Parameters (Behavioral)
2.5.1. Assessment of Body Weight
In present study, all the animals were observed for changes in the body weight before commencing the study protocol and at the end of the study (after 60 days).
2.5.2. Elevated-X Maze Test
The structure of the maze consists of two 50 cm × 10 cm open and 50 cm × 10 cm × 40 cm enclosed arms that are diverged from the central core of the platform (10 cm × 10 cm), forming a plus sign.32 The maze was wrapped with a black acrylic sheet, which was elevated above the floor level to about 50 cm using a central support. All the four arms with fitted with infrared beams placed at a regular distance. The experiment was started in the dark phase of the light cycle between 9:00 and 12:00 h. The trial was conducted by placing the rat facing toward the open arm on the central platform. During the experiment, the behavior of the rat was observed for 5 min as (i) the first entry of the rat in either of the arms, (ii) the number of access in the open or enclosed arms, and (iii) time spent by the rat in both the arms.33 The entry in the arms was noted when all of the four paws were placed on the floor. The maze was cleaned with damp and dry towels after each trial.
2.5.3. Open-Field Test
All the animals were trained for the test according to the protocol described every morning.34 A plexiglass with a wooden floor apparatus (100 cm width, 100 cm diameter, and 40 cm height) was segregated into 25 (595) squares. Upon starting the experiment, the rats were placed on the fixed position of the open field chamber every time, and their change in the behavior was recorded for 5 min using a video camera. Following observations were noted: (a) the number of square boxes travelled, only counted when the rat invades the square box with all the paws on the floor. The number of centers (nine squares) and surrounded squares (16 squares) travelled by the rat was recorded. (b) Grooming; that is, paw and fur licking and scratching the body. (c) Rearing; that is, sniffing, getting upright on rear limbs, and bending on the wall with forelimbs.35
2.5.4. Morris Maze Test
Memory impairment and cognition impairment were evaluated using the Morris water test. The test system consists of a black ring-shaped pool divided into four quadrants (168 cm diameter and 50 cm depth) filled with water (20 ± 2 °C) was bounded by visual signs of different shapes, sizes, and colurs. A black hidden circular Perspex platform was arranged 2 cm beneath the water surface in the northwest (NW) quadrant so that rats could avoid swimming.36 The rats were selected according to their swimming capacity by noting down the latency time to reach the hidden platform. During the training phase, the rats were directed to the exit of the water tank onto the platform with the help of visual signs. The rat was positioned in the water tank from the selected entry points facing opposite to the platform, and the test was repeated for 4 days (8 trials/day). The starting entry point was randomly changed during each trial, letting the rat find out the hidden platform for 60 s, and if unsuccessful to reach the platform, the rat was trained to swim toward the platform. The rat was left to hold onto the platform for around 30 s to allow visualization of the memory, marking this as the end of the experiment.37 Morris water test training was conducted between 11:00 AM and 2:00 PM using a mounted web camera to exclude variations in the rat performance.
2.6. Biochemical Analysis
2.6.1. Acetylcholinesterase Activity
Aliquots from the brain homogenates were prepared and used to measure acetylcholinesterase (AChE) activity by the ELISA plate method using the purchased AChE assay kit and measurement was performed spectrometrically at 410 nm. The AChE activity was expressed in micromoles per minute per milligram ofprotein.38,39
2.6.2. Malondialdehyde Determination
Equal quantities (2 mL) of the brain homogenate and trichloroacetic acid (10% w/v) were assorted, cooled, and centrifuged. To 0.5 mL of the supernatant, 3 mL of (0.67%) thiobarbituric acid was added, allowed to react in hot water for 15 min, and normalized for 5 min. The absorbance was measured at 535 nm on an ultraviolet (UV) spectrophotometer. The quantity of malondialdehyde (MDA) produced was expressed as nanomolar of MDA per gram of wet tissue. Activity of MDA was estimated using the molar extinction coefficient of 1.56 × 105 mol/L/cm.40,41
2.6.3. Measurement of CAT
The test mixture consists of 200 mM potassium phosphate buffer (pH 7) and 10 mM hydrogen peroxide. An alteration in the absorbance was measured at 240 nm spectrophotometrically. Activity of CAT was estimated using the molar extinction coefficient of 43.6 M–1 cm–1 of H2O2. The decomposition of 1 μmol of H2O2 per minute at a pH of 7.0 was measured for one unit of CAT.42
2.6.4. Determination of Glutathione S-Transferase
The mixture consists of 100 mM potassium phosphate buffer (pH 7) containing 1 mM glutathione, 1 mM 1-chloro-2,4-dinitrobenzene, and 60 μL of plasma. The absorbance of the reaction mixture was estimated at 340 nm. The glutathione S-transferase (GSH) activity was considered using the molar extinction coefficient of 9.6 mM–1 cm–1 of glutathione 2,4-dinitrobenzene.43
2.6.5. Measurement of Myeloperoxidase
The reaction mixture includes 100 mM phosphate buffer (pH 6.0), 0.167 mg/mL o-dianisidine dihydrochloride, 1% H2O2, and the homogenate (0.1 mL). The absorbance of the reaction mixture was recorded at 460 nm. Myeloperoxidase (MPO) activity was estimated using the molar extinction coefficient of 11.3 mM–1 cm–1 of oxidized o-dianisidine.44,45
2.6.6. Estimation of SOD
The SOD reagent consists of xanthine (0.1 mmol/L), ethylenediaminetetraacetic acid (0.1 mmol/L), bovine serum albumin (50 mg), nitro blue tetrazolium (25 mmol/L), and Na2CO3 (40 mmol/L). 50 μL of the tissue homogenate was added to 0.9 mL of the SOD reagent and 25 units of xanthine oxidase. The mixture was incubated for 25 min. The reaction was terminated by incorporating 1 mL of copper chloride (0.8 mmol/L). The absorbance of the mixture was measured at 560 nm.46,47
2.6.7. Estimation of the Nitrite Content
To 0.2 mL of the supernatant, a Griess reagent solution was added and quantified calorimetrically. The conversion of nitrite from nitrate produces a purple azo compound, which was recorded spectrophotometrically at 546 nm. The results are given as nanomolar per milligram of protein.48
2.6.8. Estimation of Neuroinflammatory Cytokines
The inflammatory cytokines such as interleukin-6 (IL-6), IL-1β, and TNF-α were estimated following the assay protocol using an ELISA kit.49
2.7. Statistical Assessment
The results of the following parameters were analyzed using one-way ANOVA, followed by Tukey’s comparison test, except for the Morris water test, which was analyzed using two-way ANOVA, followed by Bonferroni’s test, and are expressed as mean ± SEM using GraphPad prism software. The criterion of data significance was set at p < 0.05.
3. Results
3.1. Acute Toxicity Study
Acute oral toxicity studies showed that malvidin was safe up to the limit dose, that is, 2000 mg/kg b wt in rats. During the 14 days of the acute toxicity study, no morbidity or clinical appearance of symptoms were observed. Thus, based on the acute oral toxicity study data, we chose 1/20th and 1/10th doses, that is, 100 and 200 mg/kg, of malvidin for performing the main study.
3.2. Changes in the Body Weight
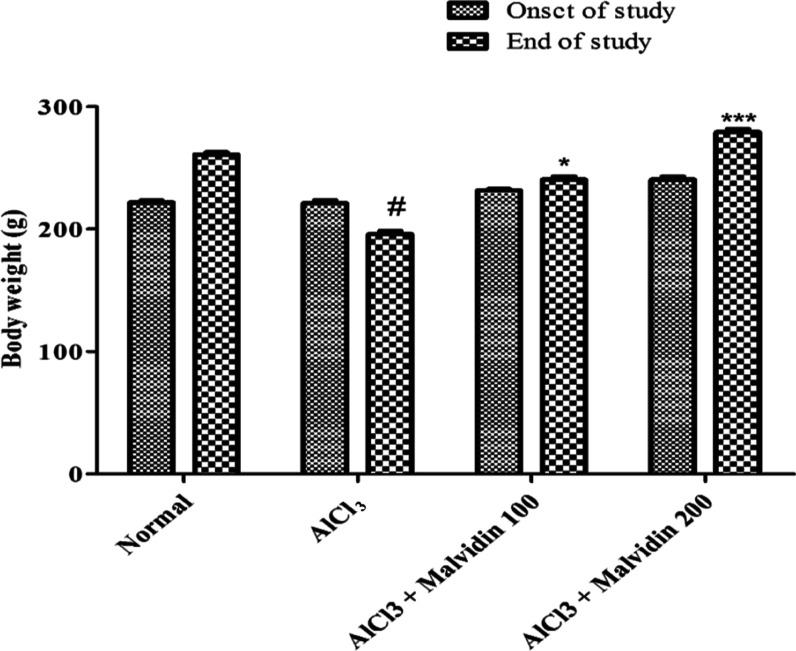
The changes in the body weight of rats were corelated prior to beginning the experiment and at the final day, on the 61st day, of the study using two-way ANOVA test. On administration of AlCl3, a marked decline in the body weight was observed. Treatment with malvidin at 100 mg/kg (p < 0.05) and 200 mg/kg (p < 0.001) doses resulted in a marked increase in the body weight at the end of the study, as shown in Figure 2.
Figure 2.
Effect of malvidin on rat body weight. All values are expressed as mean ± SD (p value < 0.05 and < 0.001 are expressed as * and ***, respectively, when compared with the disease control group); # significant as compared to the normal control group (p < 0.001). Correlation among the groups was obtained using two-way ANOVA, followed by the Bonferroni post comparison test.
3.3. Behavioral Parameters
3.3.1. Elevated X Maze Test
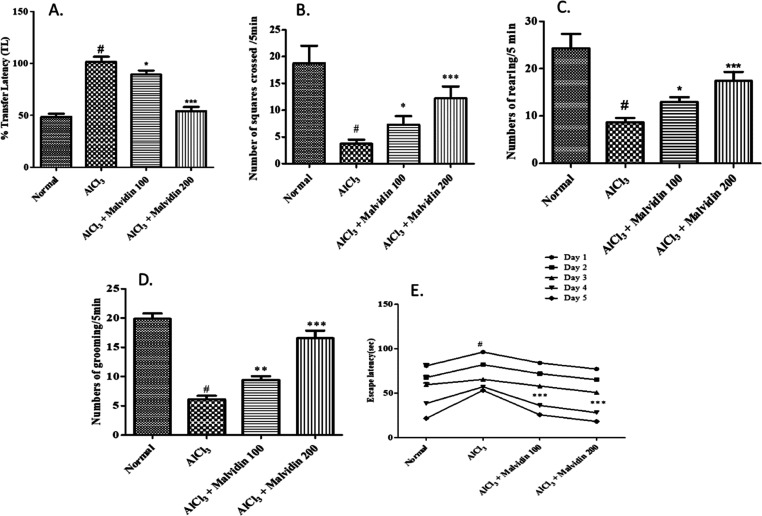
Upon evaluation on the elevated X maze test, the values of the normal control, AlCl3, and malvidin (100 and 200 mg/kg) were found to be 43.23 ± 4.5, 99 ± 5.6, 91.66 ± 4.2, and 54.33 ± 6.8 s, respectively, by one-way ANOVA test. The transfer latency in the AlCl3 group was increased as compared to that of the control group (p < 0.001). The malvidin-treated groups showed a dose-dependent decrease, but the higher-dose malvidin group (200 mg/kg) exhibited a significant decrease in contrast to that of the control (p < 0.001). (Figure 3A).
Figure 3.
Alteration in behavioral parameters in aluminum-exposed rats. (A) Percentage transfer latency; (B) number of squares crossed per 5 min; (C) number of rearing per 5 min; (D) number of grooming per 5 min; and (E) escape latency. All values are expressed as mean ± SD (p value < 0.05, 0.01, and 0.001 are expressed as *, **, and ***, respectively, when compared with the disease control group); # significant as compared to the normal control group (p < 0.001). Correlation among the groups was obtained using one-way ANOVA, followed by Tukey’s test.
3.3.2. Open-Field Test
The behavioral analysis was estimated using the post hoc test. A marked elevation (p < 0.001) in the number of squares travelled is observed as compared with that of the disease control group (3.67 ± 7.24 s) with mean values of 100 and 200 mg/kg malvidin determined to be 4.46 ± 6.2 and 8.18 ± 7.5 s, respectively. The numbers of rearing and grooming also exhibit a significant increase in the malvidin-treated groups. The mean differences of the disease control and 100 and 200 mg/kg malvidin groups were found to be as follows: number of rearing—8.91 ± 5.2, 11.76 ± 4.8, and 16.91 ± 5.6 s and number of grooming—6.01 ± 5.5, 8.98 ± 6.3, and 16.6 ± 7.3, respectively (Figure 3B–D).
3.3.3. Morris Maze Test
The Morris maze test was performed to evaluate the cognition and learning ability in rats (including all groups) for 5 days. The escape latency to search the submerged platform was shortened in the treatment groups, especially 200 mg/kg malvidin (p < 0.001), when compared to that of the disease control group. The mean differences at day 5 were significantly decreased for normal control, disease control, and 100 and 200 mg/kg malvidin groups to 21.8 ± 5.8, 53.2 ± 4.3, 25.8 ± 8.1, and 18.2 ± 4.6, respectively (Figure 3E).
3.4. Biochemical Analysis
3.4.1. AchE Determination
When compared to the normal group, the disease control group (p < 0.001) exhibited an elevation in AchE. In contrast to the disease control group, lower-dose 100 mg/kg (3.71 ± 0.6; p < 0.05) and higher-dose 200 mg/kg (3.30 ± 0.53; p < 0.001) malvidin groups resulted in a significant decrease in AchE activity, depicting that malvidin lowered the hippocampal AchE in rats (Figure 4).
Figure 4.
Effect of malvidin on acetylcholine esterase activity in aluminum-exposed rats. All values are expressed as mean ± SD (p value < 0.05 and < 0.001 are expressed as * and *** respectively, when compared with the disease control group); # significant as compared to the normal control group (p < 0.001). Correlation among the groups was obtained using one-way ANOVA, followed by Tukey’s test.
3.4.2. SOD Measurement
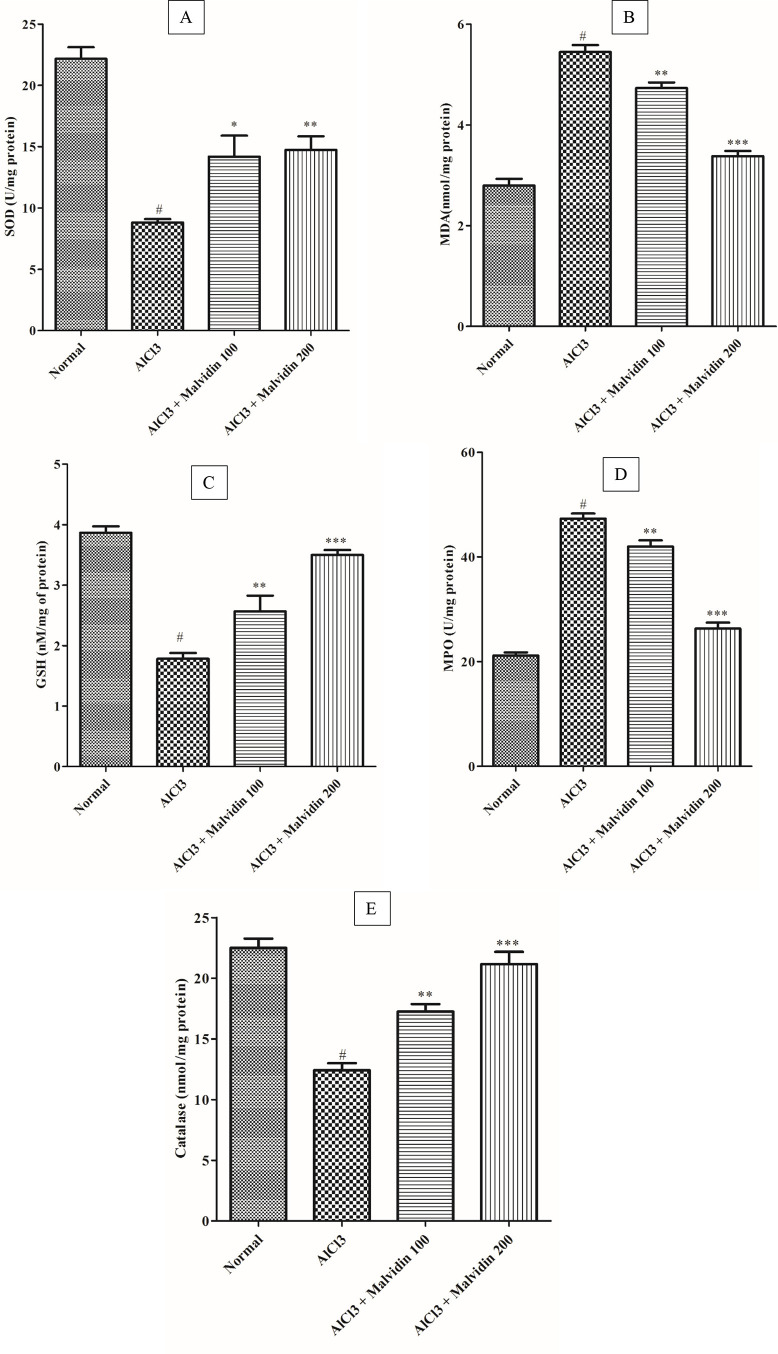
The SOD levels in the treatment groups (16.68 ± 1.3 for the 100 mg/kg group and 16.53 ± 1.5 for the 200 mg/kg groups) were elevated considerably (p < 0.05) when compared with that of the disease control group. In the AlCl3 group, the SOD level significantly reduced (p < 0.01) when compared to that of the control group (Figure 5A).
Figure 5.
Effect of malvidin on antioxidant enzyme activities in aluminum-exposed rats. (A) SOD, (B) MDA, (C) GSH, (D) MPO, and (E) CAT. All values are expressed as mean ± SD (p value < 0.05, 0.01, and 0.001 are expressed as *, **, and ***, respectively, when compared with the disease control group), # significant as compared to the normal control group (p < 0.001). Correlation among the groups was obtained using one-way ANOVA, followed by Tukey’s test.
3.4.3. MDA Determination
The MDA levels of the treatment groups 100 and 200 mg/kg malvidin showed a significant decrease (4.33 ± 0.43 and 4.23 ± 0.51, respectively; p < 0.001) when compared to that of the disease control groups. The level of MDA was increased in the disease control group as compared to that of the control group (Figure 5B).
3.4.4. GSH Estimation
A significant reduction in GSH and a subsequent elevation in both the lower dose (3.80 ± 0.70; 100 mg/kg, p < 0.001) and higher dose (3.38 ± 0.39; 200 mg/kg, p < 0.001) treatment groups, respectively, were observed as compared to that of the disease control group. A marked elevation in the MDA activity in the disease group (p < 0.01) was observed when compared to that of the normal group (Figure 5C).
3.4.5. MPO Assessment
A significant increase in the MPO activity of the disease control group (p < 0.0001) was observed when compared to that of the normal group. When compared to that of the disease group, a significant elevation in MPO activity was observed in both lower dose (36.50 ± 3.18; 100 mg/kg, p < 0.01) and higher dose (31.41 ± 3.30; 200 mg/kg, p < 0.001) malvidin-treated groups (Figure 5D).
3.4.6. Catalase
The disease control group revealed a significant decline in the CAT activity (p < 0.001) when compared to the normal group. Groups treated with malvidin with doses of 100 mg/kg (20.19 ± 2.07; p < 0.01) and 200 mg/kg (21.59 ± 2.50; p < 0.001), exhibited a remarkable decline when compared to the disease control group (Figure 5E).
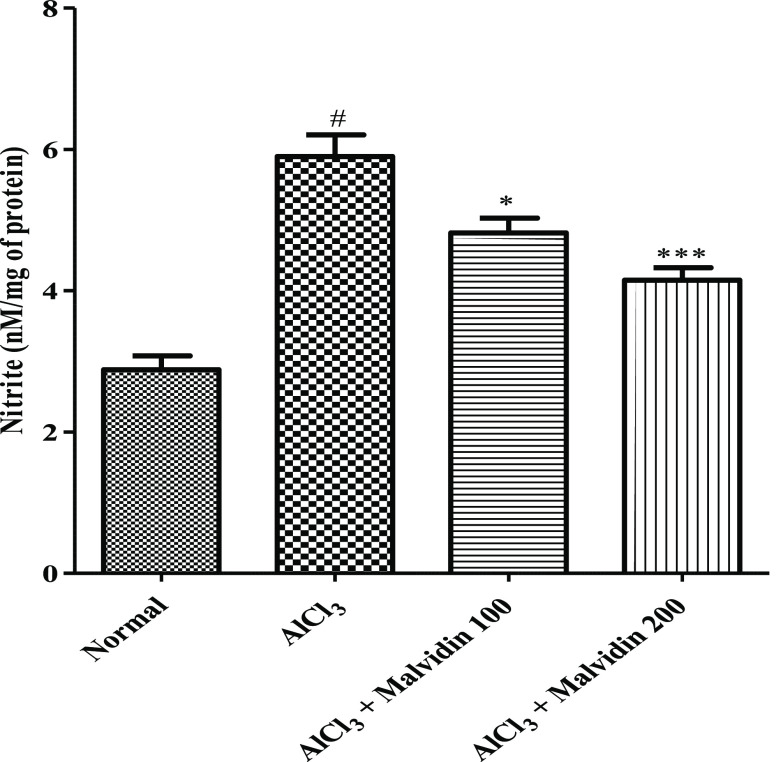
3.4.7. Determination of the Nitrite Content
The nitrite content of the disease control group is augmented remarkably (p < 0.001) when compared to that of the control group. A significant decrease in the nitrite content was observed in both treatment groups (3.53 ± 0.62; 100 mg/kg and 3.75 ± 0.49; 200 mg/kg, p < 0.001), as shown in Figure 6.
Figure 6.
Effect on nitrite content of malvidin in aluminum-exposed rats. All values are expressed as mean ± SD (p value < 0.05 and 0.001 are expressed as * and ***, respectively, when compared with the disease control group); # significant as compared to the normal control group (p < 0.001). Correlation among the groups was obtained using one-way ANOVA, followed by Tukey’s test.
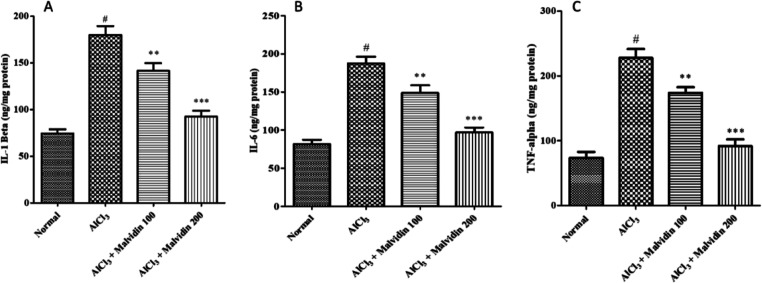
3.5. Estimation of Neuroinflammatory Cytokines
A significant decline in proinflammatory cytokines, that is, IL-6, IL-1β, and TNF-α, was observed in the disease control group as compared to that of the normal group. Both treatment groups (100 and 200 mg/kg malvidin, p < 0.001) showed a significant reduction in inflammatory cytokines IL-6 (148.3 ± 9.56 and 82.72 ± 8.08), IL-1β (140.5 ± 7.66 and 90.92 ± 7.75), and TNF-α (171.7 ± 11.29 and 95.07 ± 9.20) when compared to the disease control group, as depicted in Figure 7A–C.
Figure 7.
Effect on inflammatory cytokines of malvidin in aluminum-exposed rats. (A) IL-1β, (B) IL-6, and (C) TNF-α. All values are expressed as mean ± SD (p value < 0.05 and 0.001 are expressed as * and ***, respectively, when compared with the disease control group); # significant as compared to the normal control group (p < 0.001). Correlation among the groups was obtained using one-way ANOVA, followed by Tukey’s test.
4. Discussion
A longer exposure to elemental metals is known to be harmful and dangerous and may account for oxidative stress neurodegeneration. AlCl3 is an essential metal, but accumulation may produce neurotoxicity in the brain. Al also modifies the cholinergic transmission, affecting the acetylcholine level in the brain.50,51 Previous studies showed that chronic AlCl3 exposure is responsible for memory and cognition impairment due to alteration in the neurons.52−54
In the present investigation, malvidin alleviates the cognitive behavior, biochemical parameters, and inflammatory cytokines in AlCl3-exposed rats, indicating the antioxidant activity of malvidin, correlating various studies.55−57 A behavioral study such as the Morris maze test is the most preferred test to study the anxiety and cognition. The animals are left to search for the hidden platform, and the latent time was measured. As indicated by previous reports, AlCl3 caused memory and behavioral deficits, which was clearly observed from the study results.58,59 The behavioral parameters showed that malvidin significantly (p < 0.001) improved the memory power when compared to AlCl3. It was proven that in a dose-dependent manner, a 200 mg/kg dose of malvidin significantly (p < 0.001) enhanced the cognition impairment induced by AlCl3 in terms of the behavioral parameters tested, including the elevated X maze test, Morris maze test, and open-field test when compared to the 100 mg/kg dose of malvidin. From the results, the behavioral tests displayed that malvidin improved the motor coordination and learning abilities in AlCl3-exposed rats.
Hippocampus is linked with several excitatory and inhibitory neurotransmitters, and any damage in the neuronal transmitters can lead to neurodegenerative disorders. AchE is a crucial enzyme in the brain that is responsible for cholinergic neuronal membrane integrity and breakdown of Ach. Al exposure causes cholinergic destruction by altering the cholinergic pathway and transmission.35,60 In the present study, AlCl3 significantly escalated the AchE activity, the same as earlier findings.61 Malvidin-treated groups reduced the AchE levels, thereby increasing the level of Ach and improving the cognitive functions of the brain.
Oxidative stress generates ROS, which is the main cause for neuronal impairment, inflammation, and ultimately cell death, leading to neuroprogressive disorders. The antioxidants produced by the body naturally are the initial defense against the oxidative stress. Antioxidants slow down the oxidative stress by decreasing the free radical generation (ROS).62 CAT converts hydrogen peroxide into water and O2 through its protective mechanism to oxidative stress.63 Superoxide anions are generated by the SOD enzyme as a defense against oxidative stress. The current study showed that AlCl3 decreased the glutathione, SOD, and CAT levels in the rat brain, thereby increasing the oxidative damage, the same as that in previous studies.64,65 Malvidin treatment with a dose of 200 mg/kg displayed a more significant increase in the antioxidative parameters when compared with the 100 mg/kg malvidin treatment.
As described in previous studies, AlCl3 elevated the MDA, MPO, and nitrite contents in the rat brain.66 However, when compared to the treatment groups, a marked decrease in the antioxidant enzymes was observed, depicting the protective role against oxido-nitrosative stress. Therefore, the stated results prove the antioxidant property of malvidin and that it reduced the oxidative damage produced by AlCl3-induced neurodegeneration in rats.
Inflammatory mediators are the body’s defense system, which sense the inflamed tissues. In neuroinflammation, the NF-κB situated in the brain activates the inflammatory mediators such as IL-6, IL-1β, and TNF-α to initiate the process of modulation of inflammation.67 The study results revealed that AlCl3-exposed rats showed an increased cytokine level (TNF-α, IL-1β, and IL-6), similar to previously reported findings,68,69 whereas malvidin treatment reduced these levels significantly in both the groups. The overall findings suggest an anti-inflammatory role of malvidin against AlCl3-induced neurotoxicity in rats.
5. Conclusions
To summarize, malvidin downregulates AlCl3-induced memory impairment, cholinergic dysfunction, and the oxidative stress generated, suggesting neuroprotective action. The study showed a dose-dependent activity of malvidin, showing a significant change at the higher dose (200 mg/kg) compared to that at the lower dose (100 mg/kg). The results revealed that malvidin can be a promising therapeutic agent against neurodegenerative diseases and progression through its antioxidative mechanism.
Acknowledgments
This research project is supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Authors are thankful to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this research.
The authors declare no competing financial interest.
References
- Gandhi S.; Abramov A. Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxid. Med. Cell. Longev. 2012, 428010. 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Kukreti R.; Saso L.; Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M.; Kato-Negishi M. Link between Aluminum and the Pathogenesis of Alzheimer’s Disease: The Integration of the Aluminum and Amyloid Cascade Hypotheses. Int. J. Alzheimer’s Dis. 2011, 276393. 10.4061/2011/276393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya S.; Prakash T.; Madhu K. D.; Goli D. Multifaceted Effects of Aluminium in Neurodegenerative Diseases: A Review. Biomed. Pharmacother. 2016, 83, 746–754. 10.1016/j.biopha.2016.07.035. [DOI] [PubMed] [Google Scholar]
- Milardi D.; Rizzarelli E.. Neurodegeneration: Metallostasis and Proteostasis; Royal Society of Chemistry, 2011. [Google Scholar]
- Majumdar A.; Nirwane A.; Kamble R. Coenzyme Q10 Abrogated the 28 Days Aluminium Chloride Induced Oxidative Changes in Rat Cerebral Cortex. Toxicol. Int. 2014, 21, 214–221. 10.4103/0971-6580.139814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Gill K. D. Oxidative Stress and Mitochondrial Dysfunction in Aluminium Neurotoxicity and Its Amelioration: A Review. Neurotoxicology 2014, 41, 154–166. 10.1016/j.neuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Taïr K.; Kharoubi O.; Taïr O. A.; Hellal N.; Benyettou I.; Aoues A. Aluminium-Induced Acute Neurotoxicity in Rats: Treatment with Aqueous Extract of Arthrophytum (Hammada Scoparia). J. Acute Dis. 2016, 5, 470–482. 10.1016/j.joad.2016.08.028. [DOI] [Google Scholar]
- Sharma D. R.; Wani W. Y.; Sunkaria A.; Kandimalla R. J.; Verma D.; Cameotra S. S.; Gill K. D. Quercetin Protects Against Chronic Aluminum-Induced Oxidative Stress and Ensuing Biochemical, Cholinergic, and Neurobehavioral Impairments in Rats. Neurotox. Res. 2013, 23, 336–357. 10.1007/s12640-012-9351-6. [DOI] [PubMed] [Google Scholar]
- Cheraghi E.; Golkar A.; Roshanaei K.; Alani B. Aluminium-Induced Oxidative Stress, Apoptosis and Alterations in Testicular Tissue and Sperm Quality in Wistar Rats: Ameliorative Effects of Curcumin. Int. J. Fertil. Steril. 2017, 11, 166–175. 10.22074/ijfs.2017.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan Murali Achary V.; Jena S.; Panda K. K.; Panda B. B. Aluminium Induced Oxidative Stress and DNA Damage in Root Cells of Allium Cepa L. Ecotoxicol. Environ. Saf. 2008, 70, 300–310. 10.1016/j.ecoenv.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Tang J.; Huang L.; Shen X.; Zhang R.; Chen J. Aluminium in Food and Daily Dietary Intake Assessment from 15 Food Groups in Zhejiang Province, China. Food Addit. Contam., Part B 2016, 9, 73–78. 10.1080/19393210.2015.1135193. [DOI] [PubMed] [Google Scholar]
- Strade E.; Kalnina D.; Kulczycka J. Water Efficiency and Safe Re-Use of Different Grades of Water - Topical Issues for the Pharmaceutical Industry. Water Resour. Ind. 2020, 24, 100132. 10.1016/j.wri.2020.100132. [DOI] [Google Scholar]
- Bondy S. C. Low Levels of Aluminum Can Lead to Behavioral and Morphological Changes Associated with Alzheimer’s Disease and Age-Related Neurodegeneration. Neurotoxicology 2016, 52, 222–229. 10.1016/j.neuro.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Fulgenzi A.; Vietti D.; Ferrero M. E. Aluminium Involvement in Neurotoxicity. BioMed Res. Int. 2014, 2014, 758323. 10.1155/2014/758323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M.; Kato-Negishi M.; Hosoda R.; Imamura L.; Tsuda M.; Kuroda Y. Brain-Derived Neurotrophic Factor Protects Cultured Rat Hippocampal Neurons from Aluminum Maltolate Neurotoxicity. J. Inorg. Biochem. 2003, 97, 124–131. 10.1016/s0162-0134(03)00255-1. [DOI] [PubMed] [Google Scholar]
- Canales J. J.; Corbalán R.; Montoliu C.; Llansola M.; Monfort P.; Erceg S.; Hernandez-Viadel M.; Felipo V. Aluminium Impairs the Glutamate-Nitric Oxide-CGMP Pathway in Cultured Neurons and in Rat Brain in Vivo: Molecular Mechanisms and Implications for Neuropathology. J. Inorg. Biochem. 2001, 87, 63–69. 10.1016/s0162-0134(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Golub M. S.; Han B.; Keen C. L.; Gershwin M. E. Effects of Dietary Aluminum Excess and Manganese Deficiency on Neurobehavioral Endpoints in Adult Mice. Toxicol. Appl. Pharmacol. 1992, 112, 154–160. 10.1016/0041-008x(92)90291-y. [DOI] [PubMed] [Google Scholar]
- Bastin A.; Sadeghi A.; Nematollahi M. H.; Abolhassani M.; Mohammadi A.; Akbari H. The Effects of Malvidin on Oxidative Stress Parameters and Inflammatory Cytokines in LPS-Induced Human THP-1 Cells. J. Cell. Physiol. 2021, 236, 2790–2799. 10.1002/jcp.30049. [DOI] [PubMed] [Google Scholar]
- Seo H. R.; Choi M. J.; Choi J. M.; Ko J. C.; Ko J. Y.; Cho E. J. Malvidin Protects WI-38 Human Fibroblast Cells Against Stress-Induced Premature Senescence. J. Cancer Prev. 2016, 21, 32–40. 10.15430/jcp.2016.21.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D.-X.; Fujii M.; Terahara N.; Yoshimoto M. Molecular Mechanisms Behind the Chemopreventive Effects of Anthocyanidins. J. Biomed. Biotechnol. 2004, 2004, 321. 10.1155/s1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes F. L.; Pereira Q. C.; Zarricueta M. L.; Dos Santos R. C. Malvidin Protects against and Repairs Peptic Ulcers in Mice by Alleviating Oxidative Stress and Inflammation. Nutrients 2021, 13, 3312. 10.3390/nu13103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.; Han D.; Kim B.; Baek N.; Baik B.-K. Antioxidant and Anti-Hypertensive Activity of Anthocyanin-Rich Extracts from Hulless Pigmented Barley Cultivars. Int. J. Food Sci. Technol. 2013, 48, 984–991. 10.1111/ijfs.12050. [DOI] [Google Scholar]
- Bognar E.; Sarszegi Z.; Szabo A.; Debreceni B.; Kalman N.; Tucsek Z.; Sumegi B.; Gallyas F. Antioxidant and Anti-Inflammatory Effects in RAW264.7 Macrophages of Malvidin, a Major Red Wine Polyphenol. PLoS One 2013, 8, e65355 10.1371/journal.pone.0065355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H.; Li H.; Wan S.-P.; Zeng Q.-T.; Cheng L.-X.; Jiang L.-L.; Peng Y.-D. Cardioprotective Effects of Malvidin Against Isoproterenol-Induced Myocardial Infarction in Rats: A Mechanistic Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 2007–2016. 10.12659/msm.902196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R. D.; Wells R. W.; Hurst S. M.; McGhie T. K.; Cooney J. M.; Jensen D. J. Blueberry Fruit Polyphenolics Suppress Oxidative Stress-Induced Skeletal Muscle Cell Damage in Vitro. Mol. Nutr. Food Res. 2010, 54, 353–363. 10.1002/mnfr.200900094. [DOI] [PubMed] [Google Scholar]
- Dani C.; Pasquali M. A. B.; Oliveira M. R.; Umezu F. M.; Salvador M.; Henriques J. A. P.; Moreira J. C. F. Protective Effects of Purple Grape Juice on Carbon Tetrachloride-Induced Oxidative Stress in Brains of Adult Wistar Rats. J. Med. Food 2008, 11, 55–61. 10.1089/jmf.2007.505. [DOI] [PubMed] [Google Scholar]
- Shih P.-H.; Yeh C.-T.; Yen G.-C. Anthocyanins Induce the Activation of Phase II Enzymes through the Antioxidant Response Element Pathway against Oxidative Stress-Induced Apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. 10.1021/jf071933i. [DOI] [PubMed] [Google Scholar]
- Chacko A.; Ittiyavirah S. P. Neuroprotective Effect of Gracilaria Corticata against Aluminium-Induced Neurotoxicity in the Hippocampus and Cerebral Cortex of Rat Brain: Biochemical and Histological Approach. Asian J. Pharm. Pharmacol. 2019, 5, 604–613. 10.31024/ajpp.2019.5.3.24. [DOI] [Google Scholar]
- Abdelazem H. Effect of Moringa Oleifera on Antioxidant Enzymes and Oxidative Stress Induced by Aluminium Exposure in Male Albino Rat Testes. Int. J. Cancer Biomed. Res. 2020, 3, 34–41. 10.21608/jcbr.2019.20628.1005. [DOI] [Google Scholar]
- Wu T.; Gao Y.; Guo X.; Zhang M.; Gong L. Blackberry and Blueberry Anthocyanin Supplementation Counteract High-Fat-Diet-Induced Obesity by Alleviating Oxidative Stress and Inflammation and Accelerating Energy Expenditure. Oxid. Med. Cell. Longev. 2018, 2018, 4051232. 10.1155/2018/4051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A.-K.; Guest P. C.; Sarnyai Z.. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. In Pre-Clinical Models: Techniques and Protocols; Guest P. C., Ed.; Methods in Molecular Biology; Springer: New York, NY, 2019; pp 69–74. [DOI] [PubMed] [Google Scholar]
- Schrader A. J.; Taylor R. M.; Lowery-Gionta E. G.; Moore N. L. T. Repeated Elevated plus Maze Trials as a Measure for Tracking Within-Subjects Behavioral Performance in Rats (Rattus Norvegicus). PLoS One 2018, 13, e0207804 10.1371/journal.pone.0207804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che Y.; Zhou Z.; Shu Y.; Zhai C.; Zhu Y.; Gong S.; Cui Y.; Wang J.-F. Chronic Unpredictable Stress Impairs Endogenous Antioxidant Defense in Rat Brain. Neurosci. Lett. 2015, 584, 208–213. 10.1016/j.neulet.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Thenmozhi A. J.; Raja T. R. W.; Janakiraman U.; Manivasagam T. Neuroprotective Effect of Hesperidin on Aluminium Chloride Induced Alzheimer’s Disease in Wistar Rats. Neurochem. Res. 2015, 40, 767–776. 10.1007/s11064-015-1525-1. [DOI] [PubMed] [Google Scholar]
- Higaki A.; Mogi M.; Iwanami J.; Min L.-J.; Bai H.-Y.; Shan B.-S.; Kukida M.; Kan-no H.; Ikeda S.; Higaki J.; Horiuchi M. Predicting Outcome of Morris Water Maze Test in Vascular Dementia Mouse Model with Deep Learning. PLoS One 2018, 13, e0191708 10.1371/journal.pone.0191708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Liu Y.; Zhao J.; Xing X.; Zhang C.; Meng H. Neuroprotective Effects of D-(-)-Quinic Acid on Aluminum Chloride-Induced Dementia in Rats. J. Evidence-Based Complementary Altern. Med. 2020, 5602597. 10.1155/2020/5602597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi E.; Agrawal R.; Nath C.; Shukla R. Effect of Melatonin on Neuroinflammation and Acetylcholinesterase Activity Induced by LPS in Rat Brain. Eur. J. Pharmacol. 2010, 640, 206–210. 10.1016/j.ejphar.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V.; Featherstone R. M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Zahedi-Amiri Z.; Taravati A.; Hejazian L. B. Protective Effect of Rosa Damascena Against Aluminum Chloride-Induced Oxidative Stress. Biol. Trace Elem. Res. 2019, 187, 120–127. 10.1007/s12011-018-1348-4. [DOI] [PubMed] [Google Scholar]
- Hacioglu G.; Senturk A.; Ince I.; Alver A. Assessment of Oxidative Stress Parameters of Brain-Derived Neurotrophic Factor Heterozygous Mice in Acute Stress Model. Iran. J. Basic Med. Sci. 2016, 19, 388–393. [PMC free article] [PubMed] [Google Scholar]
- Macêdo L. G.; Carvalho-Silva M.; Ferreira G. K.; Vieira J. S.; Olegário N.; Gonçalves R. C.; Vuolo F. S.; Ferreira G. C.; Schuck P. F.; Dal-Pizzol F.; Streck E. L. Effect of Acute Administration of L-Tyrosine on Oxidative Stress Parameters in Brain of Young Rats. Neurochem. Res. 2013, 38, 2625–2630. 10.1007/s11064-013-1180-3. [DOI] [PubMed] [Google Scholar]
- Veerendra Kumar M. H.; Gupta Y. K. Effect of Centella Asiatica on Cognition and Oxidative Stress in an Intracerebroventricular Streptozotocin Model of Alzheimer’s Disease in Rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 336–342. 10.1046/j.1440-1681.2003.03842.x. [DOI] [PubMed] [Google Scholar]
- Cruz-Álvarez S.; Santana-Martínez R.; Avila-Chávez E.; Barrera-Oviedo D.; Hernández-Pando R.; Pedraza-Chaverri J.; Maldonado P. D. Apocynin Protects against Neurological Damage Induced by Quinolinic Acid by an Increase in Glutathione Synthesis and Nrf2 Levels. Neuroscience 2017, 350, 65–74. 10.1016/j.neuroscience.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Mullane K. M.; Kraemer R.; Smith B. Myeloperoxidase Activity as a Quantitative Assessment of Neutrophil Infiltration into Ischemie Myocardium. J. Pharmacol. Methods 1985, 14, 157–167. 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Haddadi R.; Rashtiani R. Anti-Inflammatory and Anti-Hyperalgesic Effects of Milnacipran in Inflamed Rats: Involvement of Myeloperoxidase Activity, Cytokines and Oxidative/Nitrosative Stress. Inflammopharmacology 2020, 28, 903–913. 10.1007/s10787-020-00726-2. [DOI] [PubMed] [Google Scholar]
- Hritcu L.; Noumedem J. A.; Cioanca O.; Hancianu M.; Kuete V.; Mihasan M. Methanolic Extract of Piper Nigrum Fruits Improves Memory Impairment by Decreasing Brain Oxidative Stress in Amyloid Beta(1–42) Rat Model of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2014, 34, 437–449. 10.1007/s10571-014-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Zhang X.; Broderick M.; Fein H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. 10.3390/s30800276. [DOI] [Google Scholar]
- Javed H.; Azimullah S.; Meeran M.; Ansari S.; Ojha S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. 10.3390/ijms20071538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Gill K. D. Aluminium Neurotoxicity: Neurobehavioural and Oxidative Aspects. Arch. Toxicol. 2009, 83, 965–978. 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- Klotz K.; Weistenhöfer W.; Neff F.; Hartwig A.; van Thriel C.; Drexler H. The Health Effects of Aluminum Exposure. Dtsch. Ärztebl. Int. 2017, 114, 653–659. 10.3238/arztebl.2017.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azib L.; Debbache-Benaida N.; Costa G. D.; Atmani-Kilani D.; Saidene N.; Ayouni K.; Richard T.; Atmani D. Pistacia lentiscus L. leaves extract and its major phenolic compounds reverse aluminium-induced neurotoxicity in mice. Ind. Crops Prod. 2019, 137, 576–584. 10.1016/j.indcrop.2019.05.062. [DOI] [Google Scholar]
- Auti S. T.; Kulkarni Y. A. Neuroprotective Effect of Cardamom Oil Against Aluminum Induced Neurotoxicity in Rats. Front. Neurol. 2019, 10, 399. 10.3389/fneur.2019.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly H. F.; Elrigal N. S.; Ali S. A.; Rizk M. Z.; Ebrahim N. A. Modulatory Effects of Casimiroa Edulis on Aluminium Nanoparticles - Associated Neurotoxicity in A Rat Model of Induced Alzheimer’s Disease. Mater. Environ. Sci. 2018, 9, 1931. [Google Scholar]
- Sun J.; Li X.; Luo H.; Ding L.; Jiang X.; Li X.; Jiao R.; Bai W. Comparative Study on the Stability and Antioxidant Activity of Six Pyranoanthocyanins Based on Malvidin-3-Glucoside. J. Agric. Food Chem. 2020, 68, 2783–2794. 10.1021/acs.jafc.9b06734. [DOI] [PubMed] [Google Scholar]
- Sun B.; Spranger I.; Yang J.; Leandro C.; Guo L.; Canário S.; Zhao Y.; Wu C. Red Wine Phenolic Complexes and Their in Vitro Antioxidant Activity. J. Agric. Food Chem. 2009, 57, 8623–8627. 10.1021/jf901610h. [DOI] [PubMed] [Google Scholar]
- Speer H.; D’Cunha N. M.; Alexopoulos N. I.; McKune A. J.; Naumovski N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. 10.3390/antiox9050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash D.; Gopinath K.; Sudhandiran G. Fisetin Enhances Behavioral Performances and Attenuates Reactive Gliosis and Inflammation During Aluminum Chloride-Induced Neurotoxicity. NeuroMolecular Med. 2013, 15, 192–208. 10.1007/s12017-012-8210-1. [DOI] [PubMed] [Google Scholar]
- Maya S.; Prakash T.; Goli D. Evaluation of Neuroprotective Effects of Wedelolactone and Gallic Acid on Aluminium-Induced Neurodegeneration: Relevance to Sporadic Amyotrophic Lateral Sclerosis. Eur. J. Pharmacol. 2018, 835, 41–51. 10.1016/j.ejphar.2018.07.058. [DOI] [PubMed] [Google Scholar]
- Al-Otaibi S. S.; Arafah M. M.; Sharma B.; Alhomida A. S.; Siddiqi N. J. Synergistic Effect of Quercetin and α-Lipoic Acid on Aluminium Chloride Induced Neurotoxicity in Rats. J. Toxicol. 2018, 2018, 2817036. 10.1155/2018/2817036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasbe P.; Jangra A.; Lahkar M. Mangiferin Ameliorates Aluminium Chloride-Induced Cognitive Dysfunction via Alleviation of Hippocampal Oxido-Nitrosative Stress, Proinflammatory Cytokines and Acetylcholinesterase Level. J. Trace Elem. Med. Biol. 2015, 31, 107–112. 10.1016/j.jtemb.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Tan B. L.; Norhaizan M. E.; Liew W.-P.-P.; Sulaiman Rahman H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtourou Y.; Aouey B.; Kebieche M.; Fetoui H. Protective Role of Naringin against Cisplatin Induced Oxidative Stress, Inflammatory Response and Apoptosis in Rat Striatum via Suppressing ROS-Mediated NF-ΚB and P53 Signaling Pathways. Chem. Biol. Interact. 2015, 239, 76–86. 10.1016/j.cbi.2015.06.036. [DOI] [PubMed] [Google Scholar]
- Bais S.; Kumari R.; Prashar Y. Ameliorative Effect of Trans-Sinapic Acid and Its Protective Role in Cerebral Hypoxia in Aluminium Chloride Induced Dementia of Alzheimer’s Type. CNS Neurol. Disord. - Drug Targets 2018, 17, 144–154. 10.2174/1871527317666180309130912. [DOI] [PubMed] [Google Scholar]
- Prakash A.; Shur B.; Kumar A. Naringin Protects Memory Impairment and Mitochondrial Oxidative Damage against Aluminum-Induced Neurotoxicity in Rats. Int. J. Neurosci. 2013, 123, 636–645. 10.3109/00207454.2013.785542. [DOI] [PubMed] [Google Scholar]
- Alghamdi B. S. A. Possible Prophylactic Anti-Excitotoxic and Anti-Oxidant Effects of Virgin Coconut Oil on Aluminium Chloride-Induced Alzheimer’s in Rat Models. J. Integr. Neurosci. 2018, 17, 593–607. 10.3233/JIN-180089. [DOI] [PubMed] [Google Scholar]
- Zou J.; Cai P.-s.; Xiong C.-m.; Ruan J.-l. Neuroprotective Effect of Peptides Extracted from Walnut (Juglans Sigilata Dode) Proteins on Aβ25-35-Induced Memory Impairment in Mice. J. Huazhong Univ. Sci. Technol., Med. Sci. 2016, 36, 21–30. 10.1007/s11596-016-1536-4. [DOI] [PubMed] [Google Scholar]
- Jangra A.; Kasbe P.; Pandey S. N.; Dwivedi S.; Gurjar S. S.; Kwatra M.; Mishra M.; Venu A. K.; Sulakhiya K.; Gogoi R.; Sarma N.; Bezbaruah B. K.; Lahkar M. Hesperidin and Silibinin Ameliorate Aluminum-Induced Neurotoxicity: Modulation of Antioxidants and Inflammatory Cytokines Level in Mice Hippocampus. Biol. Trace Elem. Res. 2015, 168, 462–471. 10.1007/s12011-015-0375-7. [DOI] [PubMed] [Google Scholar]
- Yu L.; Zhai Q.; Tian F.; Liu X.; Wang G.; Zhao J.; Zhang H.; Narbad A.; Chen W. Lactobacillus Plantarum CCFM639 Can Prevent Aluminium-Induced Neural Injuries and Abnormal Behaviour in Mice. J. Funct. Foods 2017, 30, 142–150. 10.1016/j.jff.2016.12.041. [DOI] [Google Scholar]