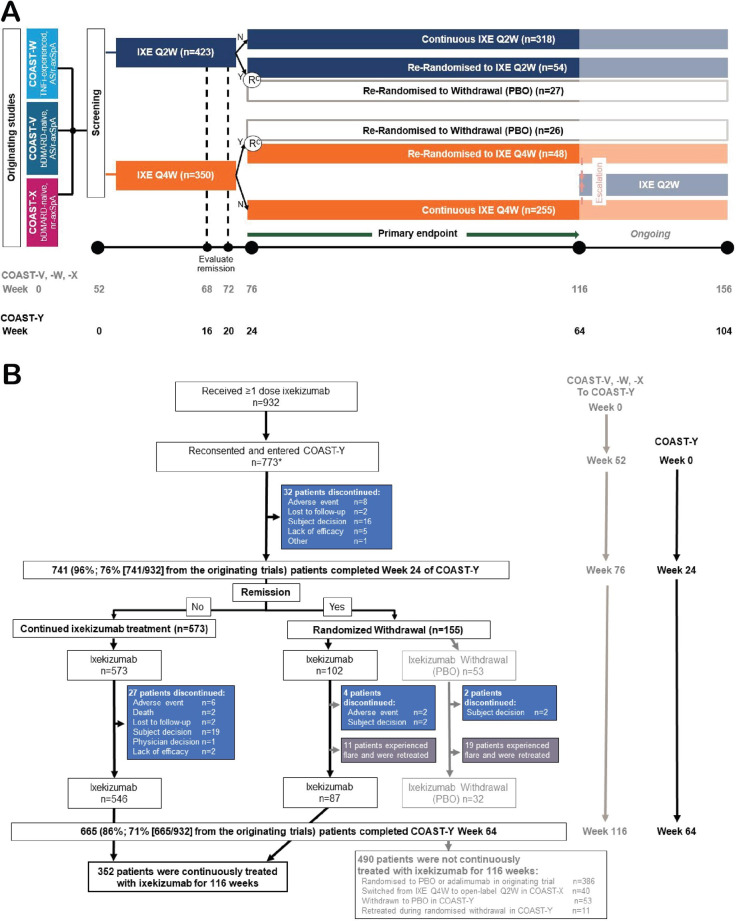
Figure 1.
Study design and patient disposition for the three originating studies of ixekizumab in axial spondyloarthritis and COAST-Y. (A) The study design shows that patients who completed the originating studies (COAST-V, COAST-W and COAST-X) and consented to enter the long-term extension study (COAST-Y) on the ixekizumab dose regimen they were receiving at the end of the originating study. Patients who completed COAST-X on placebo were started on ixekizumab every 4 weeks at the start of COAST-Y. (B) Patient disposition for COAST-Y. Disposition for the originating studies has been previously described.18–20 Efficacy analyses in this article focus on the patients who were continuously treated with ixekizumab for 116 weeks (52 weeks of the originating studies plus 64 weeks of the ongoing COAST-Y extension study). *Two patients who were originally randomised to placebo in COAST-X and remained on placebo throughout COAST-X entered COAST-Y but did not receive any ixekizumab injection, thus not included in the analysis population. axSpA, axial spondyloarthritis; bDMARD, biological disease-modifying anti-rheumatic drug; IXE, ixekizumab; n, n-number; N, no, did not achieve a state of sustained remission; nr-axSpA, non-radiographic axSpA; PBO, placebo; r-axSpA, radiographic axSpA; Y, yes, did achieve a state of sustained remission.