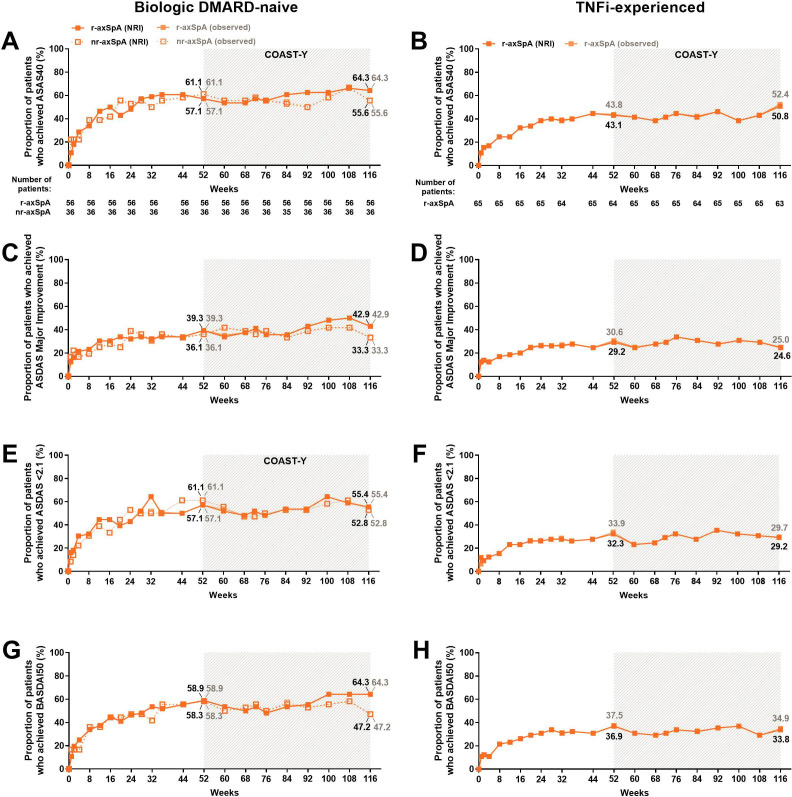
Figure 3.
Proportion of patients achieving ASAS40 and ASDAS major improvement, ASDAS <2.1 and BASDAI50 responses, for 116 weeks of IXE Q4W treatment for (A, C, E, G) biological DMARD (bDMARD)-naïve patients and (B, D, F, H) TNFi-experienced patients. Missing data among the IXE Q4W treated patients were imputed using NRI. The study was not designed to allow comparison between bDMARD-naïve patients with r-axSpA and bDMARD-naïve patients with nr-axSpA. Data are presented as the proportion (%) of patients, both NRI (black) and observed (grey) values. ASAS40, 40% improvement in Assessment of Spondyloarthritis International Society criteria; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI50, 50% improvement in Bath Ankylosing Spondylitis Disease Activity Index; DMARD, disease-modifying antirheumatic drug; IXE, ixekizumab; nr-axSpA, non-radiographic axSpA; r-axSpA, radiographic axSpA; TNFi, tumour necrosis factor inhibitor.