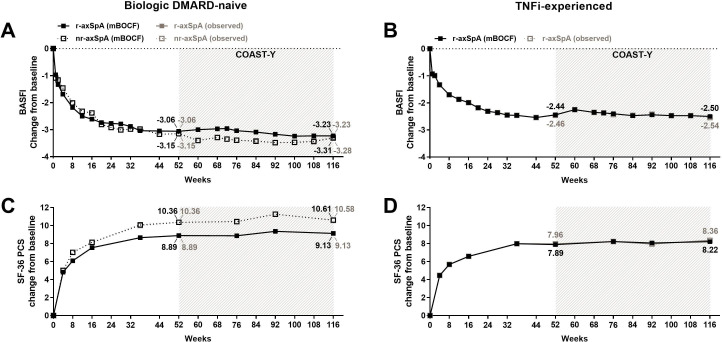
Figure 4.
Improvement of BASFI and Medical Outcomes Study 36-item Questionnaire Short-Form Health Survey (SF-36) PCS with up to 116 weeks of continuous ixekizumab treatment. For (A, C) biological DMARD (bDMARD)-naïve patients and (B, D) TNFi-experienced patients. Missing data among the patients continuously treated with ixekizumab were imputed using the mBOCF method. The study was not designed to allow comparison between bDMARD-naïve patients with r-axSpA and bDMARD-naïve patients with nr-axSpA. Data are presented as the proportion (%) of patients, both mBOCF (black) and observed (grey) values. axSpA, axial spondyloarthritis; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARD, biological disease-modifying antirheumatic drug; mBOCF, modified baseline observation carried forward; nr-axSpA, non-radiographic axSpA; PCS, Physical Component Summary; r-axSpA, radiographic axSpA; TNFi, tumour necrosis factor inhibitor.