Abstract
Objectives
Oncology surgeons use animals and cadavers in training because of a lack of alternatives. The aim of this work was to develop a design methodology to create synthetic liver models familiar to surgeons, and to help plan, teach and rehearse patient-specific cancerous liver resection surgery.
Design
Synthetic gels were selected and processed to recreate accurate anthropomorphic qualities. Organic and synthetic materials were mechanically tested with the same equipment and standards to determine physical properties like hardness, elastic modulus and viscoelasticity. Collected data were compared with published data on the human liver. Patient-specific CT data were segmented and reconstructed and additive manufactured models were made of the liver vasculature, parenchyma and lesion. Using toolmaking and dissolvable scaffolds, models were transformed into tactile duplicates that could mimic liver tissue behaviour.
Results
Porcine liver tissue hardness was found to be 23 H00 (±0.1) and synthetic liver was 10 H00 (±2.3), while human parenchyma was reported as 15.06 H00 (±2.64). Average elastic Young’s modulus of human liver was reported as 0.012 MPa, and synthetic liver was 0.012 MPa, but warmed porcine parenchyma was 0.28 MPa. The final liver model demonstrated a time-dependant viscoelastic response to cyclic loading.
Conclusion
Synthetic liver was better than porcine liver at recreating the mechanical properties of living human liver. Warmed porcine liver was more brittle, less extensible and stiffer than both human and synthetic tissues. Qualitative surgical assessment of the model by a consultant liver surgeon showed vasculature was explorable and that bimanual palpation, organ delivery, transposition and organ slumping were analogous to human liver behaviour.
Keywords: HEPATIC SURGERY, HEPATOCELLULAR CARCINOMA, SURGICAL TRAINING, COLORECTAL METASTASES
WHAT IS ALREADY KNOWN ON THIS TOPIC
Rigid, three-dimensional printed anatomy has been used for surgical planning.
Polydimethylsiloxane (PDMS) elastomers are known for their ability to emulate soft tissues.
Some mechanical properties of pig liver are comparable to human liver.
WHAT THIS STUDY ADDS
Oil-saturated PDMS mimics in vivo human liver hardness better than ex vivo pig liver.
Embedding fluid-filled, PDMS lumen ‘vasculature’ structures in solid synthetic organs can recreate complex internal anatomy.
PDMS-based surrogate organs can be used to plan and rehearse patient-specific surgical strategy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Demonstration of new production methodologies enables surgeons to teach and learn hepatic lesion removal techniques safely on a realistic surrogate organ.
Future surgeons can rehearse difficult, patient-specific dissections prior to operating on the live patient.
Use of synthetic organs in training mitigates risks posed by experiential learning.
Introduction
To remove primary or secondary cancerous liver tumours, surgeons and radiologists use imaging data and sometimes computer assistance to plan a patient-specific surgical strategy.1 2 Occasionally, a three-dimensional (3D) model of the organ might be additive manufactured (AM) to gain a better understanding of especially complex cases, but these models are rigid and cannot be used to rehearse or teach any procedures.
Patient-specific, AM 3D models are rare but are of great educational importance to surgical trainees. In the UK, trainee surgeons are required to complete 35 liver or pancreatic resections on live patients to complete their training,3 but most trainees’ first interaction with a living organ is on the operating table. So, a model that could mimic living soft tissue fidelity would offer trainee surgeons an opportunity to familiarise themselves with the sensory experience of managing live organs. With enough internal detail, these user groups might even be able to rehearse liver surgery prior to training on living patients, mitigating some of the risk associated with experiential learning.4
While there has been some work to provide alternative solutions to experiential learning using live animals, there remains a dearth of realistic models.5 Cadavers are widely considered to be the gold standard in training and simulation of surgical procedures, including liver surgery, even though liver tumours may not be presented in the donor. Costs associated with cadaver laboratory management like logistics, staffing and storage often limit availability, while venue location, religious restrictions and disposal further limit their use. In addition, many medical schools in the UK do not have a licence to use human tissues on campus.6 7
To help meet the need for alternatives to animal organs, cadavers and experiential learning, soft liver models have previously been produced. Some models focus on image guidance tuition using degradable organic compounds, but emulation of the aesthetic and tactile qualities of soft tissues was not required.8–10 In a previous attempt to recreate the liver for robotic surgical training, fabrication methodologies were explored, but characteristic organ attributes like modulus, hardness and aesthetics were not the focus of the study, so these were largely ignored.11 During surgery on any organ, the surgeon relies on sensorial feedback from the organ through touch or palpation. Embodied, tacit knowledge of organ hardness and elasticity, as well as visual cues, allow experienced surgeons to conduct their work with confidence, so any synthetic organ model should be able to mimic these qualities too. Acquiring the mechanical information needed to replicate these palpable qualities is no easy task though.
Fortunately, the hardness of in vivo human livers has been previously measured during open surgery with repeatable test standards (using a Shore 00 calibrated durometer).12 Other studies used non-contact methods to measure the elasticity and viscoelasticity of in vivo tumours.13 14 Because of the destructive nature of some contact-based, elasticity test methods, like tensile testing, ex vivo porcine liver has been used by other authors to determine the elasticity of liver parenchyma and blood vessels.15–17 However, porcine organs have been reported to be up to twice as hard as live human liver.18–20 Most studies that use ex vivo test specimens fail to mention if specimens were hydrated or prewarmed before testing, conditions well known to affect the hardness and elastic response of both organic soft tissues and synthetic elastomers.21 22
So, for any soft tissue characterisation and model to be reliable and repeatable, the same contact-based equipment and standards should be applied to biological and synthetic specimens during testing with findings compared with data in the literature. Before mechanical characterisation and comparison can commence, some suitable synthetic materials need to be identified.
A suitable family of adaptable synthetic elastomers called polydimethylsiloxanes (PDMS) are widely available as two-part liquids that, when mixed, can be poured into a mould to form almost any shape required. PDMS models have previously been shown to simulate many of the mechanical properties of soft tissues reported in the literature, like hardness and viscoelasticity.23–25 In addition to their transparency offering good pigmentation options, PDMS elastomers make ideal candidates for synthetic liver models because they can be easily altered to form a viscoelastic gel rather than a purely elastic rubber. The Optical and Biomedical Engineering Laboratory (OBEL) at the University of Western Australia recently reported that ‘tissue-mimicking phantoms’ can be created by adding controlled amounts of PDMS oils during the preparation of PDMS gels. Their research suggests that PDMS oil can reduce native PDMS elastic modulus to be within the range of healthy liver tissues reported in the biomechanical literature. Organ morphology can be reliably reproduced with well-known image processing and AM techniques to make rigid models of the parenchyma, vasculature or tumour direct from patients’ CT scan data.26–28 The gap in knowledge lies between transforming these rigid AM models into palpable, PDMS models with internal detailing.
In this paper, AM models were created based on CT data of a tumour detected in a liver with normal background parenchyma common in colorectal liver metastases, which accounts for most liver tumour resections in the UK. AM models were transformed into PDMS gels using a variety of novel, technology-led methods. Finally, to assess the proof-of-concept prototype organ fidelity, a consultant liver surgeon conducts mock surgery and offers the reader a brief qualitative summary of the experience.
Because these models mimic multiple properties of organs, they are referred to forthwith as ‘surrogate’ organs.
Methods
To generate the surrogate livers, eight key phases were required. These are delineated in the flow diagram shown in online supplemental file 1.
bmjgast-2022-000909supp002.pdf (346.4KB, pdf)
Apparatus
Hardness data were collected using a Shore 00 calibrated durometer as per the standard (Checkline, Cedarhurst, New York, USA; SN 50168) (see online supplemental appendix 1). Uniaxial tests (stress vs strain curves and elastic modulus) and multiaxial tests (unrecovered deformation and force degradation) were conducted as per the standards, using a standard tensile testing machine (Zwick/Roell Z2.5, Ulm, Germany). A Keyence VHX5000 digital microscope (Milton Keynes, UK) was used to measure all specimen thicknesses prior to mechanical testing.
bmjgast-2022-000909supp007.pdf (54.1KB, pdf)
The patient was scanned using a GE Revolution EVO 64 slice CT scanner (GE Medical Systems, Buckinghamshire, UK). Digital imaging and communications in medicine (DICOM) data were processed using a purpose-built software apparatus, Mimics Innovation Suite V.23.0 (Materialise, Leuven, Belgium). Models were AM using a fused deposition modelling platform with the Prusa Research i3 mk3s (Prusa Research a.s, Praha, Czech Republic).
Materials
Porcine liver preparation
To characterise porcine liver using the aforementioned apparatus, fresh liver was sourced from butcher’s offal (Michael Carter Fresh Foods, Nottingham, UK). The 3.6 kg liver was harvested from a large 18-month-old bacon pig within 24 hours after slaughter. Each sample was 15 mm (±2 mm) thick, cut using a serrated, circular, mechanical meat slicer and stored in a refrigeration unit prior to testing. All tests were conducted within 6 hours of specimen acquisition. Five slices were selected at random and warmed to 37°C in saline solution for 1 hour, prior to testing in a laboratory (at 21°C) (see online supplemental appendix 2). To eliminate the liver capsule membrane, known to be stiffer than the parenchymal tissue, any sample with the outer layer intact was discarded. All tests were conducted in unconfined conditions.
bmjgast-2022-000909supp008.pdf (81.5KB, pdf)
Surrogate liver preparation
PDMS gel, Platsil Gel 10,29 and PDMS oil (Neills Materials, Suffolk, UK) was prepared with a gel to oil ratio of 1:3 ratio based on starting hardness of 50 Shore hardness, data available from OBEL and observations of porcine liver tissue.
Each synthetic mixture was prepared in a plastic beaker with a wooden tongue depressor and degassed at −29 Hgm for 5 min to remove entrapped air prior to being poured into two premade gauge moulds with a depth of 15 and 2 mm. The thicker membrane was used for hardness tests, while the thinner membrane was cut into dumbbell-shaped pieces for uniaxial tests as well as discs for multiaxial tests, as per the standard. The vascular surrogate tissue was prepared for testing with the same method of selection and specimen preparation.
In mechanical hardness tests, all materials were measured with a Shore 00 calibrated durometer similar to those used in previous studies.15 The durometer was mounted to a stand (type 2 RX-OS-4H) with a combined load weight of 403 g to be exerted on each test specimen. Each specimen was tested as per the standards set out in ASTM D2240-15(2021)30 ISO 48-4:201831 and BS/ISO 23529:2016.32 The only difference in test conditions between synthetic and organic tissues was the specimen temperature.
In uniaxial tests, all specimens (porcine parenchyma and surrogate parenchyma) were cut into dumbbell-shaped pieces using an ISO37:201733 approved die stamp using specimen clamping ‘method A’ stated in the standard (ASTM D412-16(2021)34, BS ISO 5893:200235). The thickness for each surrogate specimen was collected using a digital microscope (Keyence VHX5000) at ×50 magnification to calculate the uniaxial and multiaxial properties prior to testing. The grip-to-grip separation was 25 mm; the preload was set to 0.5 N; and the test speed was 50 mm/min. All uniaxial specimens in each group were tested to failure. These specimen preparation and test methods, loads and speeds were similar to previous mechanical investigations.20 36 37
In multiaxial compression tests, specimens were prepared using the standard set out in BS EN ISO 20932-2:2020.38 Specimens were secured to the tensile testing machine using a ring clamp with a 120 mm test area (method A). Specimens were deformed at a maximum force of 5 N using a 100 mm hemispherical probe tip at 50 mm/min. Force displacement data were collected during cyclic loading and unloading (six cycles) to examine hysteresis. Force decay was collected from the fifth unloading cycle. Permanent deformation (bagging) data were collected during the sixth unloading cycle.
Imaging
DICOM data were acquired from the CT scan of a patient undergoing investigation for a solitary colorectal adenocarcinoma metastasis. Omnipaque 300 (80 mL; McKesson Europe AG, Stuttgart, Germany) intravenous contrast was administered prior to the scan, and images were acquired in the portal venous phase following bolus tracking. Axial CT images were reconstructed at 1.25 mm slice thickness and 1.25 mm slice increment. The CT demonstrated a single low attenuation lesion in segment 2 of the liver, measuring 17×15 mm, lying close to the left hepatic vein, consistent with a solitary liver metastasis.
Image processing
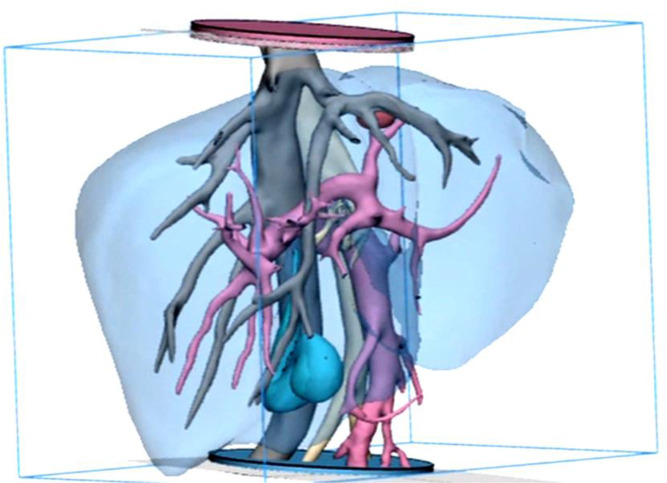
DICOM images were stacked to create a 3D digital model of the entire region. Anatomy was segmented to produce a series of volumetric digital models. A consultant radiologist (author CC) verified the digital models for accuracy, cross-referencing the CT with MRI of the patient in the same anatomical region. The liver parenchyma, hepatic artery, hepatic veins/inferior vena cava, portal vein, malignant tumour and the gall bladder were successfully identified, rendered and converted to standard tessellation language (STL) format to allow AM (figure 1).
Figure 1.
Frontal view of the final smoothed model prior to additive manufacture, showing the tumour (red), inferior vena cava (IVC) and hepatic veins (grey), hepatic artery (beige), portal vein (pink) and gall bladder (cyan), parenchyma (transparent blue). The discs at both distal ends of the model are used to fix the blood vessels in space to prevent model distortion and used as an orientation/ location marker during embedding of the surrogate vascular model. The use of hollow and dissolvable additive manufactured scaffolds for the creation of an accurate, multilayered vascular system that could be embedded in the surrogate parenchyma was previously unknown in the literature and may be useful in a variety of other applications and organs.
Additive manufacturing
The liver parenchyma and gall bladder were printed using thermoplastic aliphatic polyester, polylactic acid and smoothed by hand to remove surface construction striations.
The vascular model including the tumour were printed hollow with a 1 mm-thick lumen wall using a dissolvable, polyvinyl acetate (PVA) filament. Surface striations were smoothed using water and a soft brush.
Postprint processing and assembly
Dimensions and orientations of each AM model were proof checked against the digital models. The rigid printed tumour was removed from the vascular model, moulded and cast separately with PDMS gel saturated with 8% fibres to increase the hardness as previously described.36 The vascular model was coated in two layers of PDMS gel, the first without additives, and a second layer using 4% added fibres. The tumour surrogate was returned to its formerly marked position and the entire model was flushed with warm water overnight to remove the dissolvable PVA core, leaving behind an elastomeric ‘shell’ of the model. The open ends of the model were sealed with PDMS gel and the model was filled with PDMS oil to ensure neutral buoyancy during the embedding process. The liver parenchyma mould and locators were made with a firm PDMS rubber, Transil 20 (Neills Materials). Once cured, the AM model was cut from the mould and set aside, and a thin layer of petroleum jelly was applied to the void. The unimportant gallbladder membrane was coated with a layer of (2%) fibre-filled PDMS to make it distinguishable from the surrounding soft gels. The oil-filled vasculature was then carefully inserted into the parenchyma mould void using the markers to correctly orientate the internal model during embedding. Surrogate parenchyma (gel and oil at 1:3 ratio, with 0.4 g brown and 0.1 g red PDMS pigment per 100 g) was prepared and poured into the mould, sealed and allowed to cure overnight. Once cured, the final model was carefully removed from the mould and was ready to use.
Results
Production methodology of surrogate liver and internal vasculature was successful and overall was (mechanically) better at mimicking the living human liver than warmed (37°C, ±3°C) pig liver.
During qualitative surgical evaluation of the final model, the surrogate parenchyma was more similar to live human liver than morbid porcine liver, especially during delivery, mobility and palpation.
Hardness
A comparison between hardness (Shore 00) values given in the literature, tests on porcine/bovine tissues and tests on surrogate equivalents are shown alongside one another in figure 2.
Figure 2.
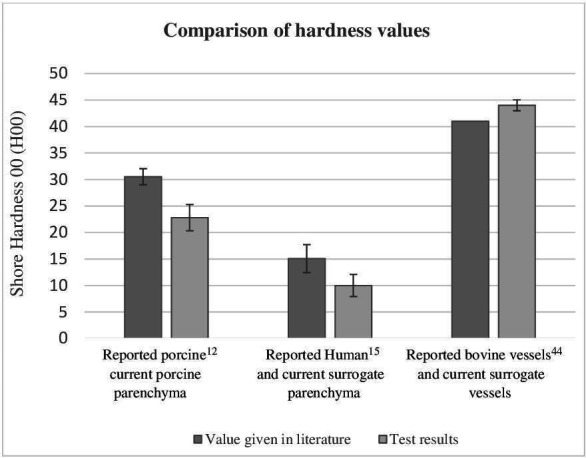
Bar chart showing the comparative hardness among 150 test specimens during indentation tests with a stand-mounted shore 00 calibrated durometer measured as per the standards. Each bar represents the arithmetic mean of 25 specimens tested. Error bars indicate an SD of 2.39. Live human liver parenchyma was previously reported as 15.06 H00 (±2.64)15 and ex vivo porcine liver was 30.52 H00±1.520.12 in the current study. Ex vivo porcine liver parenchyma was found to be 23 H00 (±2.39), and the synthetic liver parenchyma was 10 H00 (±2.09). The hardness of the surrogate vascular tissue was 44 H00 (±1.03). Hardness of porcine hepatic vascular tissue thickness did not meet requirements for test standards, but the literature assigns an aortic vascular tissue (bovine) hardness average of 41 H00.44 No specific published data could be found on the shore 00 hardness of tumours for comparison. The standard ASTM D2240-15 2021- (table x1.1), used to gather this data, specifies that the 00 shore hardness scale is the only agreed method for characterisation of both extremely soft rubber, human and animal tissues alike.
Elasticity
Literature shows the elastic Young’s modulus of healthy adult liver tissue to be between 0.01 MPa and 0.015 MPa.39 40
This investigation found porcine parenchyma to exhibit a modulus of 0.24–0.29 MPa, and the average force required to tear the specimens was 7.33 N (shown in online supplemental file 2). Surrogate parenchyma modulus was 0.009–0.014 MPa and the average force required to tear the specimens was 0.67 N (shown in online supplemental file 3) (see online supplemental appendix 2 for test condition data).
bmjgast-2022-000909supp003.pdf (97.5KB, pdf)
bmjgast-2022-000909supp004.pdf (281.4KB, pdf)
Viscoelasticity
Further mechanical characterisation of the surrogate parenchyma was carried out with a series of cyclic compression testing on five identical 145 mm×2 mm discs of surrogate liver parenchyma.38 Two types of tests were conducted to determine the viscoelastic properties. Force degradation (energy loss) is shown in online supplemental file 4, and unrecovered deformation (hysteresis) is shown in online supplemental file 5.
bmjgast-2022-000909supp005.pdf (74.7KB, pdf)
bmjgast-2022-000909supp006.pdf (2.2MB, pdf)
Discussion
Mechanical analysis
Surrogate liver tissues had similar hardness and elastic modulus to human equivalents. It was found that porcine parenchyma was more brittle, less extensible and slightly stiffer than both human and synthetic substitute tissues. Porcine liver was found to be harder than human and surrogate liver, but surrogate liver was slightly softer and more extensible than human liver overall, consistent with data in the literature.20 37 To create a harder surrogate liver, previous research has shown that the addition of short-strand fibres can be used, but its effect on other mechanical characteristics when added to oil-saturated PDMS is currently unknown and is beyond the scope of this investigation.36
When compared with human liver, the surrogate liver was found to be within the same (Young’s) modulus range but would tear with less force in tensile tests. Increasing surrogate liver modulus could be achieved by reducing the PDMS oil content, but this would also reduce the tissue slumping characteristics. Tissue slumping behaviour, exhibited by most soft organs, can be mechanically defined as relaxation and unrecovered deformation using multiaxial evaluation as shown in online supplemental files 4; 5.
Equivalent multiaxial tests with porcine organ specimens were not able to be conducted due to difficulties like cutting large enough specimens into a consistent thickness and its overall softness (deformation) under blade pressure. However, because other properties like hardness and modulus were within range, the multiaxial characterisation of the surrogates given here still offers the reader a unique, mechanical insight into what might be expected from such tests and enriches our understanding of surrogate material behaviour. A multiaxial examination provides broader mechanical data that cannot be captured through indentation or uniaxial investigation and has been used in this study to articulate typical viscoelastic behaviour of organic soft tissues. After all, multiaxial forces are applied to organs by the surgeon’s hand during palpation and by the surgeon’s tools during surgery, so such mechanical data are useful for many other studies. In robotic surgery, for instance, forces exerted by tools were shown to be relatively high (4.4–8.8 N), but the response of the tissues to these forces was unknown.41 So, to align this investigation with previous limited studies,36 the same 5 N of force was applied for multiaxial compression during cyclic tests.
The blood vessels of porcine liver could not be tested for hardness because they did not meet the eligible thicknesses required for the test standards. The hardness of bovine aortic blood vessels that are known to be thicker than both porcine and human blood vessels was previously characterised.12 Arterial tissues are also known to be stiffer than venous tissues, but because there are no published (Shore 00) hardness data specific to hepatic arterial or venous tissues, the hardness of bovine arteries was adopted for both. In addition, the equipment used in the bovine vessel study was the same as that used in the current study, allowing for direct and reliable comparative analysis.
Temperature of specimens during testing is also known to be a factor to consider when examining tissue hardness.22 So, to align our study with similar studies on living tissue,15 a saline solution bath was used to preheat porcine specimens prior to hardness tests (37°C, ±3°C). As the temperature was the same in porcine specimens as it was for the living human subjects reported in the literature, it may be assumed that at least some difference in hardness might be attributed to the lack of perfusion in the examined porcine tissue and/or morbidity of the tested porcine tissue. In contrast, surrogate liver specimens were tested at room temperature so as to consider their intended mode of use (unheated). These surrogate specimens were softer than the values given for human liver, even at room temperature. This difference may be attributable to the heterogenous macrostructure of organic soft tissues when compared with the homogenous macrostructure of synthetic surrogates. Organic counterparts contain many underlying components like blood vessels, bile ducts and cellular structures, each with their own contributary mechanical response to stress, while synthetic materials have no underlying structures. The significantly greater SD in organic test specimens also suggests that heterogeneity may have been affecting results. It may be that, lacking the microstructures and macrostructures present in organic tissues, any model’s mechanical response may never be precisely comparable to the living human liver.
Model fabrication
Regarding alignment of the components in the final model, identical locators positioned at the top and bottom of both AM models helped ensure the parenchyma model and vasculature model (with tumour) remained in their original location. A multistage moulding process that made use of layering and embedding helped to maintain accuracy and allowed for variable surrogate material properties to be used in conjunction with one another.
Aesthetically, the final model of the liver was morphologically correct because the anatomy was taken directly from AM models of patient data. Precise pigment analysis and colouration of liver tissues were less important because they have little effect on the purpose of the models’ use. Liver colouration varies widely among individuals, depending on age, disease and lifestyle, in particular, the specific colour of the patient’s liver used in this study was unknown. A generic healthy liver colour was chosen as the tissue surrounding the tumour was known to be in a healthy condition. In addition, no established colour management system exists for PDMS pigmentation, unlike well-established systems in graphics, communication systems and textiles dying, so precise colouration was solely reliant on artistic interpretation.42 43
Conclusion
This work has shown how creation, characterisation and comparison of soft tissue surrogates can be achieved by effectively combining disciplines, pooling resources and knowledge. Hardness tests were especially good at quickly revealing similarities with data given in the literature, and the standardised tests methods and equipment used make them readily accessible for future analogous investigations. Uniaxial and multiaxial test procedures requiring a wider variety of less accessible specialist equipment and specimens were more difficult to prepare, test and characterise compared with hardness testing by durometer. Data gathered during tensile tests were also more challenging to compare with similar published documentation due to variability in test methods and equipment that was often unclear in the literature. Multiaxial mechanical characterisation of soft tissues remains unexplored in the literature despite being an important descriptor of organ behaviour during palpation and surgery, perhaps due to the lack of adequate physical surrogate materials and the prevalence of mathematical modelling among biomechanical investigators. Nevertheless, this study has shown that, when combined with other test methods, multiaxial characterisation of anthropomorphic surrogates was especially good at describing the mechanical behaviour and limitations of oil saturated PDMS-based surrogates. Easy access to standards and results given here can be used to help inform the design and development of other soft tissue surrogates in the future.
From a pragmatic perspective, experimentation and development of the AM and surrogate tissue models relied heavily on the interdisciplinary experience of the authors for this study, so replication of other organs models would require similarly diversified research collaboration. For example, the fabrication of individual components and assembly of the array was exigent because of the low viscosity and slow curing time of the oil-saturated PDMS. The location and angle of entry of the premade embedded vasculature compounded these assembly issues where solutions relied heavily on the experience of the fabricators. So, successful reproduction of prototypes remains especially reliant on the experience of a suitable coalition.
Unlike actual resection surgery, cutting of the surrogate liver model only involved surgical scalpel and scissors. Assessment of multiple surrogate liver resection tools like the electrocautery laser ablator, ultrasonic aspiration dissector or the argon beam coagulator was beyond the scope of this investigation. Further development should focus on development of surrogates that simulate organic response to electrical and sonic stimuli.
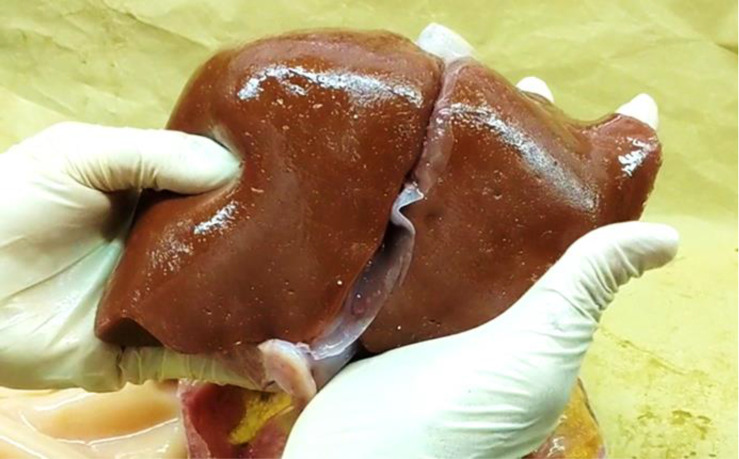
A qualitative surgical evaluation was carried out on the final model (figures 3–5) by an experienced consultant liver surgeon (author EA) (see online supplemental video 1). It was found that overall, tactile elements of the model were successful in recreating the hardness and elasticity within the force range similar to those applied during actual surgical manipulation. Forces (N) exerted during mock surgery were captured using a tensile testing machine (Zwick/Roell Z2.5) and were found to be mostly between −0.2 N and 5N for the duration of the mock surgery (see online supplemental appendix 3 for data). The mock surgery performed on the prototype model is shown in figure 5.
Figure 3.
The final prototype organ model features all the internal anatomy shown in figure 1, modified additive manufactured using the materials and methods discussed previously. The prototype surrogate organ demonstrated characteristic softness and slumping characteristics of the real organ when handled. Slumping and relaxation of the soft tissue were reflective of the real organ especially during delivery and transposition of the organ and during palpation.
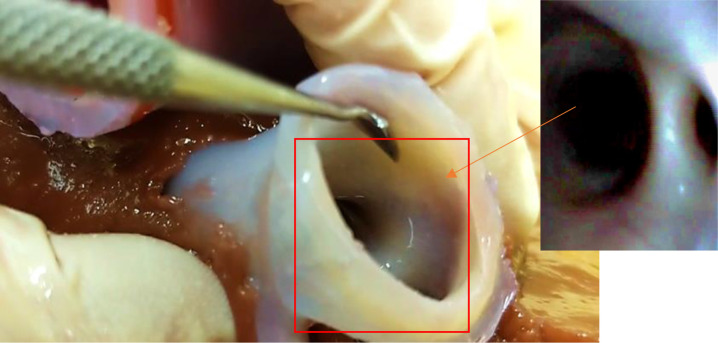
Figure 4.
View of the model’s vascular aperture taken from the upper distal end of the inferior vena cava. Insert: internal view of the hepatic artery showing the smoothness of internal vessel walls.
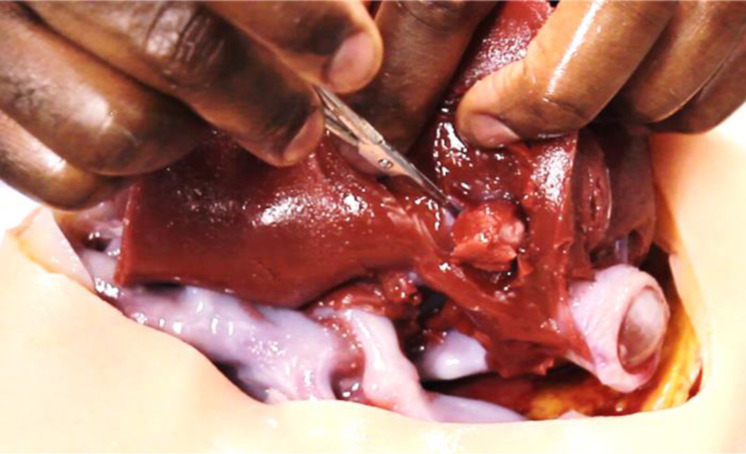
Figure 5.
Surgical rehearsal of lesion removal on prototype organ by a consultant liver surgeon, conducted without gloves to demonstrate its authenticity as a surrogate. On palpation, the liver tumour was identifiable as a discrete hard lesion on a background soft tissue with a rubbery texture. The incision reveals the tumour attached to the left hepatic vein, which ‘bled’ when the surgeon attempted removal. The procedure emulated the relationship between the tumour and major vascular structures that would be encountered during the actual procedure on the real patient, beneficial to teach surgery understudies about surgical protocol in such instances.
bmjgast-2022-000909supp001.mp4 (177.6MB, mp4)
bmjgast-2022-000909supp009.pdf (73.4KB, pdf)
Similar production methods of parenchymal tissues described here will also allow development of other surrogates with a background of cirrhotic liver parenchyma as seen in hepatocellular carcinoma (HCC). Production methods for embedding of palpable vasculature models, such as those presented in this study, are transferable to many other aspects of surgery and alternate anatomies and may be particularly useful for the creation of models for endoscopic assessment of disease or endoscopic surgery training in the future.
In summary, this work provides novel data, transferable fabrication processes and robust characterisation methods for the creation of advanced soft tissue models that will be useful for a wide variety of applications. In addition to useful surgical training opportunities, soft tissue surrogates may provide new tools for teaching forensics, prosthetics, medical device development and road traffic accident investigations.
Acknowledgments
This research was funded by the Medical Technologies and Advanced Materials Theme, School of Engineering (RD099), cofunded by the School of Art and Design (RA423) at Nottingham Trent University. The authors thank associate professor Hughes-Riley, professor Hunt and professor Dias at Nottingham Trent University, and the department of radiology staff at Queens Medical Centre, Nottingham, UK.
Footnotes
Collaborators: R Arm and A Shahidi, Advanced Textiles Research Group, Nottingham School of Art and Design, Nottingham Trent University, Nottingham, NG1 4GG, UK. C Clarke, Department of Radiology, Queens Medical Centre, Nottingham University Hospital, Nottingham, NG7 2UH, UK. E Alabraba, Department of Hepatobiliary and Pancreatic Surgery, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Derby Rd, Nottingham, NG7 2UH, UK.
Contributors: RA (the guarantor) conceptualised and prepared the original draft of the manuscript. Methodology was performed by RA, AS and CC. Mechanical evaluation was performed by RA and AS. Clinical scan evaluation was performed by CC. Data analysis was conducted by RA and AS. Data curation was performed by RA and AS. Surgical evaluation was performed by EA. Editing of the manuscript was performed by RA, AS, CC and EA.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable. Collected DICOM data were fully anonymised; permissions were granted; and ethics waivers were awarded by the relevant parties prior to data acquisition (Nottingham University Hospital, NHS).
References
- 1.Radtke A, Sotiropoulos GC, Molmenti EP, et al. Computer-assisted surgery planning for complex liver resections: when is it helpful? A single-center experience over an 8-year period. Ann Surg 2010;252:876–83. 10.1097/SLA.0b013e3181fdd012 [DOI] [PubMed] [Google Scholar]
- 2.Dong J, Yang S, Zeng J, et al. Precision in liver surgery. Semin Liver Dis 2013;33:189–203. Danish, English. 10.1055/s-0033-1351781 [DOI] [PubMed] [Google Scholar]
- 3. Anon. Joint Committee on surgical training (JCST), 2014. CCT guidelines FINAL GS V5. Available: https://www.jcst.org/search/?q=hpb
- 4.Hildebrand P, Kleemann M, Roblick U, et al. Development of a perfused ex vivo tumor-mimic model for the training of laparoscopic radiofrequency ablation. Surg Endosc 2007;21:1745–9. 10.1007/s00464-007-9216-x [DOI] [PubMed] [Google Scholar]
- 5.Scott DJ MD, Young WN, Watumull LM, et al. Development of an in vivo tumor-mimic model for learning radiofrequency ablation. J Gastrointest Surg 2000;4:620–5. 10.1016/s1091-255x(00)80112-2 [DOI] [PubMed] [Google Scholar]
- 6.Gilbody J, Prasthofer AW, Ho K, et al. The use and effectiveness of cadaveric workshops in higher surgical training: a systematic review. Ann R Coll Surg Engl 2011;93:347–52. 10.1308/147870811X582954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Act HT. legislation.gov.uk, 2004. Available: http://www.legislation.gov.uk/ukpga/2004/30/contents [Accessed May 2021].
- 8.Rethy A, Sæternes JO, Halgunset J, et al. Anthropomorphic liver phantom with flow for multimodal image-guided liver therapy research and training. Int J Comput Assist Radiol Surg 2018;13:61–72. 10.1007/s11548-017-1669-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacioni A, Carbone M, Freschi C, et al. Patient-specific ultrasound liver phantom: materials and fabrication method. Int J Comput Assist Radiol Surg 2015;10:1065–75. 10.1007/s11548-014-1120-y [DOI] [PubMed] [Google Scholar]
- 10.Lou B, Yang R, Ying P. Elasticity and echogenicity analysis of agarose phantoms mimicking liver tumors. In: Proceedings of the IEEE 32nd annual northeast bioengineering conference, 2006: 81–2. ISBN: 1629762. [Google Scholar]
- 11.Condino S, Carbone M, Ferrari V, et al. How to build patient-specific synthetic abdominal anatomies. An innovative approach from physical toward hybrid surgical simulators. Int J Med Robot 2011;7:202–13. 10.1002/rcs.390 [DOI] [PubMed] [Google Scholar]
- 12.Estermann S-J, Pahr DH, Reisinger A. Quantifying tactile properties of liver tissue, silicone elastomers, and a 3D printed polymer for manufacturing realistic organ models. J Mech Behav Biomed Mater 2020;104:103630. 10.1016/j.jmbbm.2020.103630 [DOI] [PubMed] [Google Scholar]
- 13.Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol 2007;52:1565–76. 10.1088/0031-9155/52/6/002 [DOI] [PubMed] [Google Scholar]
- 14.Barnes SC, Lawless BM, Shepherd DET, et al. Viscoelastic properties of human bladder tumours. J Mech Behav Biomed Mater 2016;61:250–7. 10.1016/j.jmbbm.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 15.Yoon YC, Lee JS, Park SU, et al. Quantitative assessment of liver fibrosis using shore durometer. Ann Surg Treat Res 2017;93:300–4. 10.4174/astr.2017.93.6.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umale S, Chatelin S, Bourdet N, et al. Experimental in vitro mechanical characterization of porcine Glisson's capsule and hepatic veins. J Biomech 2011;44:1678–83. 10.1016/j.jbiomech.2011.03.029 [DOI] [PubMed] [Google Scholar]
- 17.Chen Q, Wang Y, Li Z-Y. Re-Examination of the mechanical anisotropy of porcine thoracic aorta by uniaxial tensile tests. Biomed Eng Online 2016;15:167. 10.1186/s12938-016-0279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Srinivasan M. Characterisation of Viscoelastic Soft Tissue Properties from In vivo Animal Experiments and Inverse FE Parameter Estimation. Medical Image Computing and Computer-Assisted Intervention. In: Duncan y J, Gerig G, eds. 8Th International Conference, palm springs, Ca, USA, proceedings, part II. 3750. Springer Berlin / Heidelberg, 2005: 599–606. [DOI] [PubMed] [Google Scholar]
- 19.Snedeker JG, Barbezat M, Niederer P, et al. Strain energy density as a rupture criterion for the kidney: impact tests on porcine organs, finite element simulation, and a baseline comparison between human and porcine tissues. J Biomech 2005;38:993–1001. 10.1016/j.jbiomech.2004.05.030 [DOI] [PubMed] [Google Scholar]
- 20.Uehara H. A study on the mechanical properties of the kidney, liver, and spleen, by means of tensile stress test with variable strain velocity. Journal of Kyoto Prefectural University of Medicine 1995;104:439–51. 10.1016/j.jbiomech.2004.05.030 [DOI] [Google Scholar]
- 21.Kerdok AE, Ottensmeyer MP, Howe RD. Effects of perfusion on the viscoelastic characteristics of liver. J Biomech 2006;39:2221–31. 10.1016/j.jbiomech.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 22.Fung Y. Biomechanics: mechanical properties of living tissues. 2nd edition. New York: Springer, 1993: 269–71. [Google Scholar]
- 23.Arruda EM, Boyce MC. A three-dimensional constitutive model for the large stretch behavior of rubber elastic materials. J Mech Phys Solids 1993;41:389–412. 10.1016/0022-5096(93)90013-6 [DOI] [Google Scholar]
- 24.Boyce MC, Arruda EM. Constitutive models of rubber elasticity: a review. Rubber Chem Technol 2000;73:504–23. 10.5254/1.3547602 [DOI] [Google Scholar]
- 25.Palchesko R, Zhang L, Sun Y. Development of polydimethylsiloxane substrates with tuneable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE 2012;12:e51499. 10.1371/journal.pone.0051499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fotheringham P. 3D printing for cancer treatment – radiation therapy liver phantom. 3D Heals; Blog, Expert’s Corner. [Internet], 2019. 3DHEALS LLC. Available: https://3dheals.com/3d-printing-for-cancer-treatment-radiation-therapy-liver-phantom [Accessed 20 Nov 2021].
- 27.Öpik R, Hunt A, Ristolainen A. Development of high-fidelity liver and kidney phantom organs for use with robotic surgical systems. 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics 2012:425–30. 10.1109/BioRob.2012.6290831 [DOI] [Google Scholar]
- 28.Ahmad MS, Suardi N, Shukri A, et al. Dynamic hepatocellular carcinoma model within a liver phantom for multimodality imaging. Eur J Radiol Open 2020;7:100257. 10.1016/j.ejro.2020.100257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corp® Polytek Dev’. PlatSilGels Technical Bulletin Polytek- Soft, Translucent, RTV Liquid Silicone Rubbers for Theatrical Prosthetics, Lifecasting & Mold Making Applications Technical Bulletin. [Internet], 2017. Mouldlife.net. Available: https://www.mouldlife.net/ekmps/shops/mouldlife/resources/Other/gel-00-10-data.pdf [Accessed 04 Feb 2022].
- 30.. ASTM D2240-15 Standard Test Method for Rubber Property- Durometer Hardness [Internet]. West Conshohocken, PA. Astm.org: ASTM International; 2022. https://www.astm.org/d2240-15.html [Accessed 04 Feb 2022]. [Google Scholar]
- 31.ISO 48-4:2018 Rubber, vulcanized or thermoplastic- Determination of hardness. Part 4: Indentation hardness by durometer method [Internet], 2022. International organization for standardization. Available: https://www.iso.org/standard/74969.html [Accessed 04 Feb 2022].
- 32.ISO 23529:2016 General procedures for preparing and conditioning test pieces for physical test methods [Internet], 2016. International organization for standardization. Available: https://www.iso.org/standard/70323.html [Accessed 04 Feb 2022].
- 33.ISO 37:2017 rubber, vulcanized or thermoplastic- determination of tensile stress-strain properties [Internet], 2017. International organization for standardization. Available: https://www.iso.org/standard/68116.html [Accessed 04 Feb 2022].
- 34.ASTM D412-16(2021) . Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers- Tension, [Internet]. West Conshohocken, PA. Astm.org. ASTM International; 2021. https://www.astm.org/d0412-16r21.html [Accessed 04 Feb 2022]. [Google Scholar]
- 35.ISO 5893:2019/AMD 1:2020. rubber and plastics test equipment- tensile, flexural and compression types (constant rate of traverse) Specification amendment 1 [Internet], 2020. International organization for standardization. Available: https://www.iso.org/standard/80532.html [Accessed 04 Feb 2022].
- 36.Arm R, Shahidi A, Dias T. Mechanical behaviour of silicone membranes saturated with short strand, loose polyester fibres for prosthetic and rehabilitative surrogate skin applications. Materials 2019;12:3647. 10.3390/ma12223647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemper AR, Santago AC, Stitzel JD, et al. Biomechanical response of human liver in tensile loading. Ann Adv Automot Med 2010;54:15–26. [PMC free article] [PubMed] [Google Scholar]
- 38.ISO 20932-2:2020 Determination of the elasticity of fabrics- Part 2: Multiaxial tests. [Internet], 2020. International organization for standardization. Available: https://www.iso.org/standard/69490.html [Accessed 04 Feb 2022].
- 39.Samur E, Sedef M, Basdogan C, et al. A robotic indenter for minimally invasive characterization of soft tissues. Int Congr Ser 2005;1281:713–8. 10.1016/j.ics.2005.03.117 [DOI] [PubMed] [Google Scholar]
- 40.Ottensmeyer M, Kerdok A, Howe R. The effects of testing environment on the viscoelastic properties of soft tissues. In: Cotin S, Metaxas D, eds. Medical simulation. 3078. ISMS. Lecture Notes in Computer Science, 2004: 9–18. [Google Scholar]
- 41.Madhani J, Niemeyer G, Salisbury J. The black falcon: a teleoperated surgical instrument for minimally invasive surgery. Proceedings. 1998 IEEE/RSJ International conference on intelligent robots and systems. Innovations in theory, practice and applications. 1998;2:936–44. 10.1109/IROS.1998.727320 [DOI] [Google Scholar]
- 42.Hayden SL, Oulton DP. The quantification of small visual colour differences. Journal of the Society of Dyers and Colourists 1994;110:104–11. 10.1111/j.1478-4408.1994.tb01620.x [DOI] [Google Scholar]
- 43.Javoršek D, Javoršek A. Colour management in digital textile printing. Coloration Technology 2011;127:235–9. 10.1111/j.1478-4408.2011.00304.x [DOI] [Google Scholar]
- 44.Maclean C, Brodie R, Nash D. Shore 00 hardness measurement of bovine aorta and mock vessel materials for endovascular device design. [Internet], 2019. www.bssm.org. Available: http://www.bssm.org/uploadeddocuments/Conf%202017/2017%20papers/88_Craig_Maclean_formatted.pdf [Accessed 04 Feb 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2022-000909supp002.pdf (346.4KB, pdf)
bmjgast-2022-000909supp007.pdf (54.1KB, pdf)
bmjgast-2022-000909supp008.pdf (81.5KB, pdf)
bmjgast-2022-000909supp003.pdf (97.5KB, pdf)
bmjgast-2022-000909supp004.pdf (281.4KB, pdf)
bmjgast-2022-000909supp005.pdf (74.7KB, pdf)
bmjgast-2022-000909supp006.pdf (2.2MB, pdf)
bmjgast-2022-000909supp001.mp4 (177.6MB, mp4)
bmjgast-2022-000909supp009.pdf (73.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.