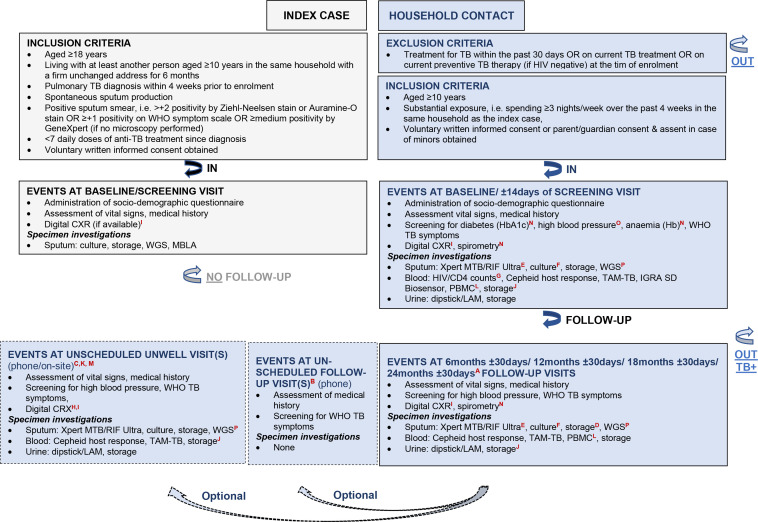
Figure 3.
Eligibility criteria and schedules of events for index cases and household contacts. A=depending on the time point of study enrolment and consequently on the duration available for follow-up, that is, 18 or 24 months, the follow-up visit at 24 months±30 days may be conditional; B=the follow-up visit by phone may be conducted after the last scheduled follow-up visit at 18 months±30 days or 24 months ±30 days to assess whether symptoms suggestive of TB have occurred, TB diagnosis has been made or anti-TB treatment has been initiated; C=unwell visits by phone or on-site may be conducted between scheduled follow-up visits if a participant presents at a recruitment healthcare facility with signs and symptoms suggestive of TB; D=coached spontaneous or induced sputum collection for storage at scheduled follow-up visit at 18 months±30 days or 24 months ±30 days, and for repetition of HIV testing if tested negative at baseline; E=coached spontaneous or induced sputum collection on the decision of the investigating team for testing by Xpert MTB/RIF Ultra if participant presents with signs and symptoms suggestive of TB; F=coached spontaneous or induced sputum collection in case of Xpert MTB/RIF Ultra positivity or strong clinical suspicion of TB for repetition of the Xpert MTB/RIF Ultra; G=in case of HIV positivity to be followed by the assessment of CD4 counts; H=CXR to be conducted at an unscheduled on-site unwell visit on the decision of the investigating team depending on the nature of symptoms reported, and the time elapsed since the last CXR including its findings; I=not to be conducted among pregnant women; J=stored venous blood includes 6 mL EDTA blood for whole blood and plasma, 4 mL serum and 2.5 mL PAXgene blood, all samples will be deep frozen for retrospective testing using new diagnostics as described in text; K=in case the evaluation of symptoms of a participant unable to present at a recruitment healthcare facility is required an unscheduled on-site or home visit will be arranged by phone, the resolution of symptoms can alternatively be addressed by phone; L=collection of PBMC at baseline and follow-up visit at 6 months±30 days is optional, thus will not be performed at each participating site and for each participant; M=in case the evaluation of symptoms of a participant unable to present at a recruitment healthcare facility is required or doubtful if required an unscheduled unwell visit by phone will be arranged, the resolution of symptoms can alternatively be addressed by phone; N=spirometry and/or diabetes (HbA1c) will be performed at scheduled follow-up visits at 6 months±30 days, 12 months ±30 days and 18 or 24 months±30 days if required or not performed at baseline, anaemia (Hb) will be performed at baseline and scheduled follow-up visits at 6 months±30 days, 12 months ±30 days and 18 or 24 months±30 days if possible; O=blood pressure measurement will be performed at baseline and scheduled follow-up visits at 6 months±30 days, 12 months ±30 days and 18 or 24 months±30 days; P=WGS to be performed once Mtb infection is confirmed and an isolate could be recovered. CD4, cluster of differentiation 4; CXR, chest radiograph; Hb, haemoglobin; HbA1c, glycated haemoglobin; IGRA, interferon gamma release assay; LAM, lioparabinomannan; MTB, Mycobacterium tuberculosis; MBLA, molecular bacterial load assay; PBMC, peripheral blood mononuclear cell; RIF, rifampicin; TAM-TB, T- cell activation marker tuberculosis; TB, tuberculosis; WGS, whole genome sequencing.