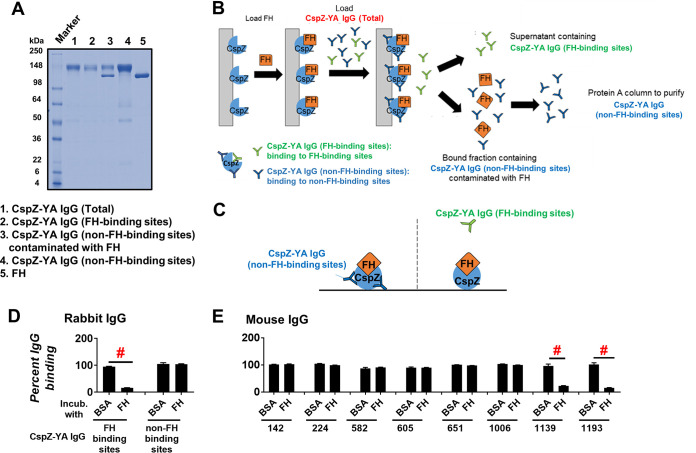
FIG 1.
IgGs that recognize both FH- and non-FH-binding sites of CspZ can be isolated separately from CspZ-YA-immunized rabbits and mice. (A) The integrity and purity assessment of CspZ-YA IgGs using SDS-PAGE. (B) A schematic diagram showing the purification process to retrieve CspZ-YA IgG (FH-binding sites) and CspZ-YA IgG (non-FH-binding sites). CspZ-conjugated resin was incubated with FH followed by loading CspZ-YA IgG (total). The bound and unbound fractions contain CspZ-YA IgG (non-FH-binding sites) and CspZ-YA IgG (FH-binding sites) IgG, respectively. After the bound fraction was eluted, the CspZ-YA IgG (non-FH-binding sites) was further purified using Protein A resin to remove contaminated FH. (C) A schematic diagram showing the experimental setup of panels D and E. (D and E) One microgram of CspZ was coated on ELISA plate wells, which were then incubated with human FH (500 nM), BSA (control), or PBS (control, data not shown), followed by the treatment of each of the CspZ-YA IgG samples. These IgGs include (D) CspZ-YA rabbit polyclonal IgGs that recognize the FH-binding site of CspZ (FH-binding sites) or non-FH-binding site (non-FH-binding sites) or (E) CspZ-YA mouse monoclonal IgGs (50 nM). The levels of bound CspZ-YA rabbit polyclonal and mouse monoclonal IgG were determined using HRP-conjugated goat anti-rabbit IgG (Sigma-Aldrich) and goat anti-mouse IgG (Sigma-Aldrich), respectively. Data were expressed as the percent IgG binding, derived by normalizing the levels of bound IgG from FH- or BSA-treated wells to that in PBS-treated wells. Data shown are the mean ± standard error of the mean (SEM) of the percent IgG binding from three independent experiments. # indicates the statistical significance (P < 0.05, Mann-Whitney test) of different levels of percent IgG binding between indicated groups.