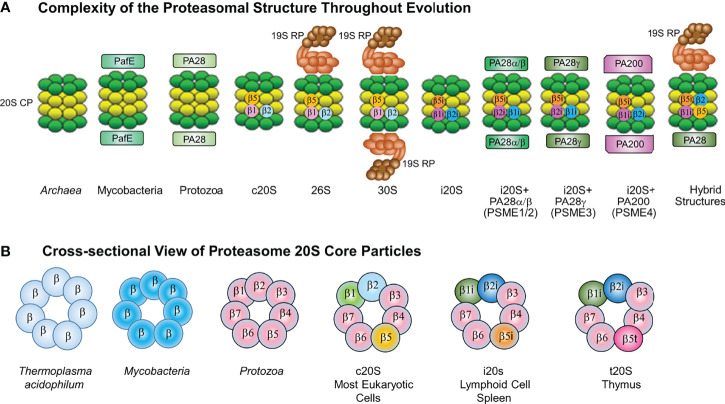
Figure 1.
(A) Complexity of the Proteasome Structure Throughout Evolution. The constitutive 20S proteasome is assembled in a (green) and b (yellow) heptameric rings. Constitutive 20S proteasomes (c20S) contain the catalytic subunits b1, b2 and b5. The 20S immunoproteasome (i20S) catalytic core contains three inducible b subunits named b1i, b2i and b5i. immunoproteasomes As previously described, intracellular proteasomes can exist in multiple different forms (Gomes, 2013). Both c20S and i20S proteasomes are found with or without regulators. The c20S is capped at one or both ends by a 19S RP (light brown) to form 26S or 30S proteasomes. The i20S proteasome is capped by either the PA28ab or PA28g complex at either one or both ends. Hybrid proteasome complexes are also found when the catalytic core is simultaneously associated with 19S RP and another type of regulator, e.g., PA28ab, PA28g, PA200. In the cytoplasm ECM29 and PSMF1 can modify the assembly or activity of the proteasome; while in the nucleus PA200 can regulate proteasome activity. (B) Cross-sectional View of Proteasome 20S Core Particles. Shown is a cross-sectional side view of 20S proteasomes showing the evolutionarily conserved barrel-like (a7b7b7a7) structure in eubacterial and eukaryotic proteasomes. Archaea and Mtb proteasomes are depicted as having seven identical b subunits, whereas eukaryotic proteasomes have seven different b subunits. In lymphoid cells and spleen, constitutive 20S proteasome catalytic subunits b1, b2 and b5 are substituted by three inducible b subunits named b1i, b2i and b5i to generate 20S proteasomes. 26S proteasomes exist with either one or two 19S caps, immunoproteasomes containing one or two 11S caps, proteasomes containing the 20S proteasome with one or two PA200 caps (in the nucleus only), and hybrid proteasomes which contain different combinations of 20S and activators.